Nitrosyl bromide
Appearance
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
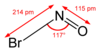
| NOBr | |||
| Molar mass | 109.910 g mol−1 | ||
| Appearance | Red gas | ||
| Boiling point | 14.5 °C (58.1 °F; 287.6 K) | ||
Refractive index (nD)
|
1.524 | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitrosyl bromide, is the chemical compound with the chemical formula NOBr. It is a red gas with a condensing point just below room temperature.
Nitrosyl bromide can be formed by the reversible reaction of nitric oxide with bromine. This reaction is of interest as it is one of very few third order homogenous gas reactions. It is prone to photodisassociation at standard pressure and temperature.