Thionyl chloride

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Sulfurous dichloride
| |
| Other names
Thionyl dichloride
Sulfurous oxychloride Sulfinyl chloride sulfinyl dichloride Dichlorosulfoxide Sulfur oxide dichloride Sulfur monoxide dichloride Sulfuryl(IV) chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.863 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 1836 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SOCl2 | |
| Molar mass | 118.97 g/mol |
| Appearance | clear; colorless to yellow odorous liquid |
| Density | 1.638 g/cm3, liquid |
| Melting point | −104.5 °C |
| Boiling point | 74.6 °C |
| reacts | |
| Solubility | soluble in benzene, chloroform, CCl4 |
Refractive index (nD)
|
1.517 (20 °C) [1] |
| Viscosity | 0.6 cP |
| Structure | |
| pyramidal | |
| 1.4 D | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl chloride, SO2Cl2, but the properties of these compounds differ significantly. Approximately 45,000 tons per year of SOCl2 were produced in the early 1990s.[2]
Properties and structure
The molecule SOCl2 is pyramidal, indicating the presence of a lone pair of electrons on the sulfur(IV) center. In contrast, the stoichiometrically related COCl2 is planar. SOCl2 reacts with water to release hydrogen chloride and sulfur dioxide.
- SOCl2 + H2O → 2 HCl + SO2
Production
The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride:[3]
- SO3 + SCl2 → SOCl2 + SO2
Other methods include syntheses from phosphorus pentachloride, chlorine, or phosgene:
- SO2 + PCl5 → SOCl2 + POCl3
- SO2 + Cl2 + SCl2 → 2 SOCl2
- SO3 + Cl2 + 2 SCl2 → 3 SOCl2
- SO2 + COCl2 → SOCl2 + CO2
The first of the above four reactions also affords phosphorus oxychloride (phosphoryl chloride), which resembles thionyl chloride in many of its reactions.
Applications
Thionyl chloride is mainly used in the industrial production of organochlorine compounds, which are often intermediates in pharmaceuticals and agrichemicals.
Organic chemistry
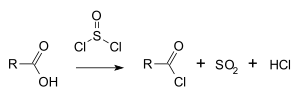
Thionyl chloride is widely used in organic synthesis. For some applications, it requires purification.[4] Classically, it converts carboxylic acids to acyl chlorides:[5]
Alcohols react with thionyl chloride to give the corresponding alkyl chlorides in Darzens reaction.[6] This reaction proceeds via an internal nucleophilic substitution.
It is preferred over other reagents such as phosphorus pentachloride because the products of the thionyl chloride reactions, HCl and SO2, are gaseous, which simplifies the purification of the product. Excess thionyl chloride can be readily removed by distillation.
Sulfonic acids react with thionyl chloride to produce sulfonyl chlorides.[7][8] Sulfonyl chlorides have also been prepared from the direct reaction of the corresponding diazonium salt with thionyl chloride.[9] Likewise, thionyl chloride will transform sulfinic acids into sulfinyl chlorides[10] and phosphonic acids into phosphoryl chlorides. Thionyl chloride will react with primary formamides to form isocyanides.[11] Amides will react with thionyl chloride to form imidoyl chlorides. However, primary amides under heating with thionyl chloride will continue on to form nitriles.[12]
Thionyl chloride can also produce nitriles from amides via E2 elimination.[13]
Inorganic chemistry
Anhydrous metal chlorides may be obtained from hydrated metal chlorides by refluxing in freshly distilled thionyl chloride:[14]
- MCln·xH2O + x SOCl2 → MCln + x SO2 + 2x HCl
Other applications
Thionyl chloride is a component of lithium-thionyl chloride batteries, where it acts as the positive electrode (cathode) with lithium as the negative electrode (anode).
Safety
SOCl2 is a reactive compound that can explosively release dangerous gases upon contact with water and other reagents. Industrial production of thionyl chloride is controlled under the Chemical Weapons Convention, where it is listed in Schedule 3. Thionyl chloride is used in the "di-di" method of producing G-series nerve agents.
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Hans-Dietrich Lauss, Wilfried Steffens “Sulfur Halides” in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a25_623
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 820. ISBN 978-0-08-022057-4..
- ^ Friedman, L. and Wetter, W. P., "Purification of Thionyl Chloride", J. Chem. Soc. A, 1967, 36-8.doi:10.1039/J19670000036
- ^ Allen, C. F. H.; Byers, Jr., J. R.; Humphlett, W. J. (1963). "Oleoyl chloride". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 739.; Rutenberg, M. W.; Horning, E. C. (1963). "1-Methyl-3-ethyloxindole". Organic Syntheses{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 620. - ^ Mondanaro, K. R.; Dailey, W. P. (2004). "3-Chloro-2-(chloromethyl)-1-propene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 10, p. 212.; Krakowiak, K. E.; Bradshaw, J. S. (1998). "4-Benzyl-10,19-diethyl-4,10,19-triaza-1,7,13,16-tetraoxacycloheneicosane". Organic Syntheses{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 34.; Feng Xu, Bryon Simmons, Robert A. Reamer, Edward Corley, Jerry Murry, and David Tschaen (2008). "Chlorination/Cyclodehydration of Amino Alcohols with SOCl2: An Old Reaction Revisited". J. Org. Chem. 73 (1): 312–5. doi:10.1021/jo701877h. PMID 18052293.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weinreb, S. M.; Chase, C. E.; Wipf, P.; Venkatraman, S. (2004). "2-Trimethylsilylethanesulfonyl chloride (SES-Cl)". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 10, p. 707. - ^ Hazen, G. G.; Bollinger, F. W.; Roberts, F. E.; Russ, W. K.; Seman, J. J.; Staskiewicz, S. (1998). "4-Dodecylbenzenesulfonyl azides". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 400. - ^ Philip J. Hogan and Brian G. Cox (2009). "Aqueous Process Chemistry: The Preparation of Aryl Sulfonyl Chlorides". Org. Process Res. Dev. 13 (5): 875–879. doi:10.1021/op9000862.
- ^ Hulce, M.; Mallomo, J. P.; Frye, L. L.; Kogan, T. P.; Posner, G. H. (1990). "(S)-(+)-2-(p-toluenesulfinyl)-2-cyclopentenone: Precursor for enantioselective synthesis of 3-substituted cyclopentanones". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 7, p. 495.; Kurzer, F. (1963). "p-Toluenesulfinyl chloride". Organic Syntheses; Collected Volumes, vol. 4, p. 937. - ^ Niznik, G. E.; Morrison, III, W. H.; Walborsky, H. M. (1988). "1-d-Aldehydes from organometallic reagents: 2-methylbutanal-1-d". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 751. - ^ Krynitsky, J. A.; Carhart, H. W. (1963). "2-Ethylhexanonitrile". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 436. - ^ John E. McMurry (2010). Fundamentals of Organic Chemistry (7th ed.). Cengage Learning. p. 767. ISBN 1-4390-4971-8.
- ^ Alfred R. Pray, Richard F. Heitmiller, Stanley Strycker (1990). "Anhydrous Metal Chlorides". Inorganic Syntheses. Inorganic Syntheses. 28: 321–323. doi:10.1002/9780470132593.ch80. ISBN 978-0-470-13259-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link)