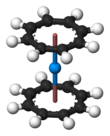
Uranocene
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bis(η8-cyclooctatetraenyl)uranium(IV)
| |||
| Other names
Uranium cyclooctatetraenide
U(COT)2 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C16H16U | |||
| Molar mass | 446.33 g/mol | ||
| Appearance | green crystals[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
pyrophoric, radioactive, and toxic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating elements from the actinide series. It is the most studied bis[8]annulene-metal system, although it has no known practical applications.[2]
Synthesis, structure and bonding[edit]
Uranocene was first described in 1968 by the group of Andrew Streitwieser, when it was prepared by the reaction of dipotassium cyclooctatetraenide and uranium tetrachloride in THF at 0°C:[1]
Uranocene is highly reactive toward oxygen, being pyrophoric in air but stable to hydrolysis. The x-ray crystal structure of uranocene was first elucidated by the group of Ken Raymond.[3] Considering the molecule to be U4+(C8H82−)2, the η8-cyclooctatetraenide groups are planar, as expected for a ring containing 10 π-electrons, and are mutually parallel, forming a sandwich containing the uranium atom. In the solid state, the rings are eclipsed, conferring D8h symmetry on the molecule. In solution the rings rotate with a low activation energy.
The uranium-cyclooctatetraenyl bonding was shown by photoelectron spectroscopy to be primarily due to mixing of uranium 6d orbitals into ligand pi orbitals and therefore donation of electronic charge to the uranium, with a smaller such interaction involving the uranium (5f)2 orbitals.[4] Electronic theory calculations agree with this result[5][6] and point out that the weaker interaction of the open-shell 5f orbitals with the ligand orbitals determines |MJ|, the magnitude of the angular momentum quantum number along the 8-fold symmetry axis of the ground state.[6]
Spectroscopic properties[edit]
Uranocene is paramagnetic. Its magnetic susceptibility is consistent with values of 3 or 4 for |MJ|, with the accompanying magnetic moment being affected by the spin-orbit coupling.[7] Its NMR spectrum is consistent with an |MJ| value of 3.[8] Electronic theory calculations from the simplest[9] to the most accurate[10] also give |MJ| values of 3 for the ground state and 2 for the first excited state, corresponding to double-group symmetry designations[11] of E3g and E2g for these states.
The green color of uranocene is due to three strong transitions in its visible spectrum.[1][12] In addition to finding vibrational frequencies, Raman spectra indicate the presence of a low-lying (E2g) excited electronic state.[12][13] On the basis of calculations,[6] the visible transitions are assigned to transitions primarily of 5f-to-6d nature, giving rise to E2u and E3u states.
Analogous compounds[edit]
Analogous compounds of the form M(C8H8)2 exist for M = (Nd, Tb, Yb, Th, Pa, Np, and Pu). Extensions include the air-stable derivative U(C8H4Ph4)2 and the cycloheptatrienyl species [U(C7H7)2]−.[2] In contrast, bis(cyclooctatetraene)iron has a very different structure, with one each of a η6- and η4-C8H8 ligands.
References[edit]
- ^ a b c Streitwieser, A.; Mueller-Westerhoff, U. (1968). "Bis(cyclooctatetraenyl)uranium (uranocene). A new class of sandwich complexes that utilize atomic f orbitals". J. Am. Chem. Soc. 90 (26): 7364. doi:10.1021/ja01028a044.
- ^ a b Seyferth, D. (2004). "Uranocene. The First Member of a New Class of Organometallic Derivatives of the f Elements". Organometallics. 23 (15): 3562–3583. doi:10.1021/om0400705.
- ^ Zalkin, Allan; Raymond, Kenneth N. (1969). "Structure of di-.pi.-cyclooctatetraeneuranium (uranocene)". Journal of the American Chemical Society. 91 (20): 5667–5668. doi:10.1021/ja01048a055. ISSN 0002-7863.
- ^ Clark, J. P.; Green, J. C. (1977). "An Investigation of the Electronic Structure of Bis(eta-cyclo-octatetraene)-actinoids by Helium-(I) and -(II) Photoelectron Spectroscopy". J. Chem. Soc., Dalton Trans. (5): 505–508. doi:10.1039/DT9770000505.
- ^ Roesch, N.; Streitwieser, A. (1983). "Quasirelativistic SCF-Xalpha Scattered-Wave Study of Uranocene, Thorocene, and Cerocene". J. Am. Chem. Soc. 105 (25): 7237–7240. doi:10.1021/ja00363a004.
- ^ a b c Chang, A. H. H.; Pitzer, R. M. (1989). "Electronic Structure and Spectra of Uranocene". J. Am. Chem. Soc. 111 (7): 2500–2507. doi:10.1021/ja00189a022.
- ^ Karraker, D. G.; Stone, J. A.; Jones, E. R.; Edelstein, N. (1970). "Bis(cyclooctatetraenyl)neptunium(IV) and Bis(cyclooctatetraenyl)plutonium(IV)". J. Chem. Phys. 92 (16): 4841–4845. doi:10.1021/ja00719a014.
- ^ Fischer, R. D. (1979). "NMR Spectroscopy of Organometallic Compounds of the f-Elements: Practical Applications". In Marks, T. J.; Fischer, R. D. (eds.). Volume 44 – Organometallics of the f-Elements. NATO Advanced Study Institutes Series: Series C – Mathematical and Physical Sciences. Dordrecht, Holland: Reidel. pp. 337–377. ISBN 90-277-0990-4.
- ^ Hayes, R. G.; Edelstein, N. (1972). "An Elementary Molecular Orbital Calculation on U(C8H8)2 and Its Application to the Electronic Structure of U(C8H8)2, Np(C8H8)2. and Pu(C8H8)2". J. Am. Chem. Soc. 94 (25): 8688–8691. doi:10.1021/ja00780a008.
- ^ Liu, W.; Dolg, M.; Fulde, P. (1997). "Low-lying electronic states of lanthanocenes and actinocenes M(C8H8)2 (M=Nd, Tb, Yb, U)". J. Chem. Phys. 107 (9): 3584–3591. Bibcode:1997JChPh.107.3584L. doi:10.1063/1.474698.
- ^ Herzberg, G. (1966). Molecular Spectra and Molecular Structure III. Electronic Spectra and Electronic Structure of Polyatomic Molecules. Princeton, New Jersey: D. Van Nostrand. p. 566.
- ^ a b Dallinger, R. F.; Stein, P.; Spiro, T. G. (1978). "Resonance Raman Spectroscopy of Uranocene: Observation of an Anomalously Polarized Electronic Band and Assignment of Energy Levels". J. Am. Chem. Soc. 100 (25): 7865–7870. doi:10.1021/ja00493a013.
- ^ Hager, J. S.; Zahardis, J.; Pagni, R. M.; et al. (2004). "Raman under nitrogen. The high-resolution Raman spectroscopy of crystalline uranocene, thorocene, and ferrocene". J. Chem. Phys. 120 (6): 2708–2718. Bibcode:2004JChPh.120.2708H. doi:10.1063/1.1637586. PMID 15268415.
Further reading[edit]
- The f elements, Nikolas Kaltsoyannis and Peter Scott. ISBN 0-19-850467-5
- Chemistry of the Elements, N. N. Greenwood and A. Earnshaw. ISBN 0-08-022057-6
- Lanthanides & Actinides: Organoactinides