User:Lottamiata/sandbox
Groups containing oxygen[edit]
Compounds that contain C-O bonds each possess differing reactivity based upon the location and hybridization of the C-O bond, owing to the electron-withdrawing effect of sp² hybridized oxygen and the donating effects of sp³ hybridized oxygen.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Ether | Ether | ROR' | alkoxy- | alkyl alkyl ether | Diethyl ether (Ethoxyethane) | |
| Alcohol | Hydroxyl | ROH | 
|
hydroxy- | -ol | 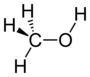 Methanol |
| Peroxide | Peroxy | ROOR | 
|
peroxy- | alkyl peroxide | Di-tert-butyl peroxide |
| Hydroperoxide | Hydroperoxy | ROOH | hydroperoxy- | alkyl hydroperoxide | Methyl ethyl ketone peroxide | |
| Ketone | Carbonyl | RCOR' | 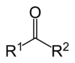
|
keto-, oxo- | -one | 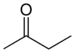 Methyl ethyl ketone (Butanone) |
| Aldehyde | Aldehyde | RCHO | 
|
aldo- | -al |  Acetaldehyde (Ethanal) |
| Acyl halide | Haloformyl | RCOX | 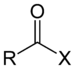
|
haloformyl- | -oyl halide |  Acetyl chloride (Ethanoyl chloride) |
| Ester | Ester | RCOOR' | 
|
alkyl alkanoate | Ethyl butyrate (Ethyl butanoate) | |
| Carboxylic acid | Carboxyl | RCOOH | 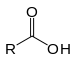
|
carboxy- | -ic acid | 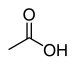 Acetic acid (Ethanoic acid) |
| Carboxylate | Carboxylate | RCOO− | 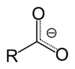 |
carboxy- | -oate | Sodium acetate (Sodium ethanoate) |
| Carbonate | Carbonate ester | ROCOOR | alkyl carbonate |
