Reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an electron recipient (called the oxidizing agent, oxidant, oxidizer, or electron acceptor).
Examples of substances that are common reducing agents include hydrogen, the alkali metals, formic acid,[1] oxalic acid,[2] and sulfite compounds.
In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/donates electrons", that "is oxidized", and that "reduces" is called the reducer or reducing agent, while the agent whose oxidation state decreases, that "gains/accepts/receives electrons", that "is reduced", and that "oxidizes" is called the oxidizer or oxidizing agent.
For example, consider the overall reaction for aerobic cellular respiration:
- C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
The oxygen (O2) is being reduced, so it is the oxidizing agent. The glucose (C6H12O6) is being oxidized, so it is the reducing agent.
Characteristics
[edit]Consider the following reaction:
The reducing agent in this reaction is ferrocyanide ([Fe(CN)6]4−). It donates an electron, becoming oxidized to ferricyanide ([Fe(CN)6]3−). Simultaneously, that electron is received by the oxidizer chlorine (Cl
2), which is reduced to chloride (Cl−
).
Strong reducing agents easily lose (or donate) electrons. An atom with a relatively large atomic radius tends to be a better reductant. In such species, the distance from the nucleus to the valence electrons is so long that these electrons are not strongly attracted. These elements tend to be strong reducing agents. Good reducing agents tend to consist of atoms with a low electronegativity, which is the ability of an atom or molecule to attract bonding electrons, and species with relatively small ionization energies serve as good reducing agents too.[citation needed]
The measure of a material's ability to reduce is known as its reduction potential.[3] The table below shows a few reduction potentials, which can be changed to oxidation potentials by reversing the sign. Reducing agents can be ranked by increasing strength by ranking their reduction potentials. Reducers donate electrons to (that is, "reduce") oxidizing agents, which are said to "be reduced by" the reducer. The reducing agent is stronger when it has a more negative reduction potential and weaker when it has a more positive reduction potential. The more positive the reduction potential the greater the species' affinity for electrons and tendency to be reduced (that is, to receive electrons). The following table provides the reduction potentials of the indicated reducing agent at 25 °C. For example, among sodium (Na), chromium (Cr), cuprous (Cu+) and chloride (Cl−), it is Na that is the strongest reducing agent while Cl− is the weakest; said differently, Na+ is the weakest oxidizing agent in this list while Cl is the strongest.[citation needed]
| Oxidizing agent | Reducing agent | Reduction Potential (V) | |
|---|---|---|---|
| Li+ + e− | ⇌ | Li | −3.04 |
| Na+ + e− | Na | −2.71 | |
| Mg2+ + 2 e− | Mg | −2.38 | |
| Al3+ + 3 e− | Al | −1.66 | |
| 2 H2O (l) + 2 e− | H2 (g) + 2 OH− | −0.83 | |
| Cr3+ + 3 e− | Cr | −0.74 | |
| Fe2+ + 2 e− | Fe | −0.44 | |
| 2 H+ + 2 e− | H2 | 0.00 | |
| Sn4+ + 2 e− | Sn2+ | +0.15 | |
| Cu2+ + e− | Cu+ | +0.16 | |
| Ag+ + e− | Ag | +0.80 | |
| Br2 + 2 e− | 2 Br− | +1.07 | |
| Cl2 + 2 e− | 2 Cl− | +1.36 | |
| MnO−4 + 8 H+ + 5 e− | Mn2+ + 4 H2O | +1.49 | |
| F2 + 2 e− | 2 F− | +2.87 |
Common reducing agents include metals potassium, calcium, barium, sodium and magnesium, and also compounds that contain the hydride H− ion, those being NaH, LiH,[5] LiAlH4 and CaH2.
Some elements and compounds can be both reducing or oxidizing agents. Hydrogen gas is a reducing agent when it reacts with non-metals and an oxidizing agent when it reacts with metals.
- 2 Li(s) + H2(g) → 2 LiH(s)[a]
Hydrogen (whose reduction potential is 0.0) acts as an oxidizing agent because it accepts an electron donation from the reducing agent lithium (whose reduction potential is -3.04), which causes Li to be oxidized and hydrogen to be reduced.
- H2(g) + F2(g) → 2 HF(g)[b]
Hydrogen acts as a reducing agent because it donates its electrons to fluorine, which allows fluorine to be reduced.
Importance
[edit]Reducing agents and oxidizing agents are the ones responsible for corrosion, which is the "degradation of metals as a result of electrochemical activity".[3] Corrosion requires an anode and cathode to take place. The anode is an element that loses electrons (reducing agent), thus oxidation always occurs in the anode, and the cathode is an element that gains electrons (oxidizing agent), thus reduction always occurs in the cathode. Corrosion occurs whenever there's a difference in oxidation potential. When this is present, the anode metal begins deteriorating, given there is an electrical connection and the presence of an electrolyte.[citation needed]
Examples of redox reaction
[edit]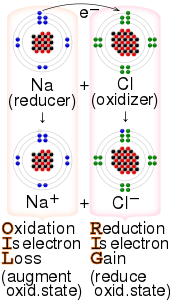
Historically, reduction referred to the removal of oxygen from a compound, hence the name 'reduction'.[7] An example of this phenomenon occurred during the Great Oxidation Event, in which biologically−produced molecular oxygen (dioxygen (O2), an oxidizer and electron recipient) was added to the early Earth's atmosphere, which was originally a weakly reducing atmosphere containing reducing gases like methane (CH4) and carbon monoxide (CO) (along with other electron donors)[8] and practically no oxygen because any that was produced would react with these or other reducers (particularly with iron dissolved in sea water), resulting in their removal. By using water as a reducing agent, aquatic photosynthesizing cyanobacteria produced this molecular oxygen as a waste product.[9] This O2 initially oxidized the ocean's dissolved ferrous iron (Fe(II) − meaning iron in its +2 oxidation state) to form insoluble ferric iron oxides such as Iron(III) oxide (Fe(II) lost an electron to the oxidizer and became Fe(III) − meaning iron in its +3 oxidation state) that precipitated down to the ocean floor to form banded iron formations, thereby removing the oxygen (and the iron). The rate of production of oxygen eventually exceeded the availability of reducing materials that removed oxygen, which ultimately led Earth to gain a strongly oxidizing atmosphere containing abundant oxygen (like the modern atmosphere).[10] The modern sense of donating electrons is a generalization of this idea, acknowledging that other components can play a similar chemical role to oxygen.
The formation of iron(III) oxide;
- 4Fe + 3O2 → 4Fe3+ + 6O2− → 2Fe2O3
In the above equation, the Iron (Fe) has an oxidation number of 0 before and 3+ after the reaction. For oxygen (O) the oxidation number began as 0 and decreased to 2−. These changes can be viewed as two "half-reactions" that occur concurrently:
- Oxidation half reaction: Fe0 → Fe3+ + 3e−
- Reduction half reaction: O2 + 4e− → 2 O2−
Iron (Fe) has been oxidized because the oxidation number increased. Iron is the reducing agent because it gave electrons to the oxygen (O2). Oxygen (O2) has been reduced because the oxidation number has decreased and is the oxidizing agent because it took electrons from iron (Fe).
Common reducing agents
[edit]This section needs additional citations for verification. (October 2016) |
- Lithium aluminium hydride (Li Al H4), a very strong reducing agent
- Red-Al (NaAlH2(OCH2CH2OCH3)2), a safer and more stable alternative to lithium aluminum hydride
- Hydrogen without or with a suitable catalyst; e.g. a Lindlar catalyst
- Sodium amalgam (Na(Hg))
- Sodium-lead alloy (Na + Pb)
- Zinc amalgam (Zn(Hg)) (reagent for Clemmensen reduction)
- Diborane
- Sodium borohydride (Na BH4)
- Ferrous compounds that contain the Fe2+ ion, such as iron(II) sulfate
- Stannous compounds that contain the Sn2+ ion, such as tin(II) chloride
- Sulfur dioxide (sometimes also used as an oxidizing agent), Sulfite compounds
- Dithionates, e.g. Na2S2O6
- Thiosulfates, e.g. Na2S2O3 (mainly in analytical chemistry)[11]
- Iodides, such as potassium iodide (K I) (mainly in analytical chemistry)
- Hydrogen peroxide (H
2O
2) – mostly an oxidant but can occasionally act as a reducing agent, typically in analytical chemistry[citation needed] - Hydrazine (Wolff-Kishner reduction)
- Diisobutylaluminium hydride (DIBAL-H)
- Oxalic acid (C
2H
2O
4) - Formic acid (HCOOH)
- Ascorbic acid (C6H8O6)
- Reducing sugars, such as erythrose, see Aldose
- Phosphites, hypophosphites, and phosphorous acid
- Dithiothreitol (DTT) – used in biochemistry labs to avoid SS-bonds
- Carbon monoxide (CO)
- Cyanides in hydrochemical metallurgical processes
- Carbon (C)
- Tris-2-carboxyethylphosphine hydrochloride (TCEP)
See also
[edit]- Corrosion – Gradual destruction of materials by chemical reaction with its environment
- Electrochemistry – Branch of chemistry
- Electrolyte – Ionic solids whose dissociation in water frees up ions carrying the electrical current in solution
- Electron acceptor – Chemical entity capable of accepting electrons
- Electron donor – Chemical entity capable of donating electrons to another entity
- Electrosynthesis – Synthesis of chemical compounds in an electrochemical cell
- Organic reduction – Redox reaction that takes place with organic compounds
- Oxidizing agent – Chemical compound used to oxidize another substance in a chemical reaction
- Redox – Chemical reaction in which oxidation states of atoms are changed
- Reducing equivalent – Chemical reaction in which oxidation states of atoms are changed
- Salt-free reduction
Notes
[edit]- ^ Half reactions: 2 Li0(s) → 2 Li+(s) + 2 e− ::::: H20(g) + 2 e− → 2 H−(g)
- ^ Half reactions: H20(g) → 2 H+(g) + 2 e− ::::: F20(g) + 2 e− → 2 F−(g)
References
[edit]- ^ Garron, Anthony; Epron, Florence (2005). "Use of formic acid as reducing agent for application in catalytic reduction of nitrate in water". Water Research. 39 (13): 3073–3081. Bibcode:2005WatRe..39.3073G. doi:10.1016/j.watres.2005.05.012. PMID 15982701.
- ^ "Oxidizing and Reducing Agents". Purdue University.
- ^ a b "Electrode Reduction and Oxidation Potential Values". www.EESemi.com. Retrieved 12 July 2021.
- ^ "Standard Electrode Potentials". hyperphysics.phy-astr.gsu.edu. Retrieved 29 March 2018.
- ^ Aufray M, Menuel S, Fort Y, Eschbach J, Rouxel D, Vincent B (2009). "New Synthesis of Nanosized Niobium Oxides and Lithium Niobate Particles and Their Characterization by XPS Analysis" (PDF). Journal of Nanoscience and Nanotechnology. 9 (8): 4780–4789. doi:10.1166/jnn.2009.1087. PMID 19928149. Archived from the original (PDF) on 2020-07-29. Retrieved 2019-09-24.
- ^ "Metals". Bitesize. BBC. Archived from the original on 2022-11-03.
- ^ Olson, Maynard V. "oxidation-reduction reaction". Britannica. Retrieved 3 May 2022.
In his Traité élémentaire de chimie, he clearly established that combustion consists of a chemical combination between oxygen from the atmosphere and combustible matter [...]. By the end of the century, his ideas were widely accepted and had been successfully applied to the more complex processes of respiration and photosynthesis. Reactions in which oxygen was consumed were classified as oxidations, while those in which oxygen was lost were termed reductions.
- ^ Kasting, J.F. (2014). "Modeling the Archean Atmosphere and Climate". Treatise on Geochemistry. Elsevier. pp. 157–175. doi:10.1016/b978-0-08-095975-7.01306-1. ISBN 9780080983004.
- ^ Buick, Roger (August 27, 2008). "When did oxygenic photosynthesis evolve?". Philosophical Transactions of the Royal Society B. 363 (1504): 2731–2743. doi:10.1098/rstb.2008.0041. ISSN 0962-8436. PMC 2606769. PMID 18468984.
- ^ Sosa Torres, Martha E.; Saucedo-Vázquez, Juan P.; Kroneck, Peter M.H. (2015). "Chapter 1, Section 2: The rise of dioxygen in the atmosphere". In Kroneck, Peter M.H.; Sosa Torres, Martha E. (eds.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences volume 15. Vol. 15. Springer. pp. 1–12. doi:10.1007/978-3-319-12415-5_1. ISBN 978-3-319-12414-8. PMID 25707464.
- ^ "Cathodic Stripping Voltammetric Procedure for Determination of Some Inorganic Arsenic Species in Water, Soil and Ores Samples".
Further reading
[edit]- "Chemical Principles: The Quest for Insight", Third Edition. Peter Atkins and Loretta Jones p. F76
External links
[edit]- Table summarizing strength of reducing agents at the Wayback Machine (archived June 11, 2011)
