Trisodium citrate
| |
| Names | |
|---|---|
| IUPAC names
Trisodium citrate
Trisodium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Other names
Citrosodine
Citric acid, trisodium salt Sodium citrate E331 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.614 |
| E number | E331iii (antioxidants, ...) |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
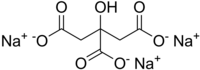
| Na3C6H5O7 | |
| Molar mass | 258.06 g/mol (anhydrous), 294.10 g/mol (dihydrate) |
| Appearance | White crystalline powder |
| Density | 1.7 g/cm3 |
| Melting point | >300 °C hydrates lose water ca. 150 C |
| Boiling point | Decomposes |
| Pentahydrate form: 92 g/100 g H2O (25 °C)[1] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1548 mg/kg (intraperitoneal, rat)[2] |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as sodium citrate, though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor.
Applications
Food
Sodium citrate is chiefly used as a food additive, usually for flavor or as a preservative. Its E number is E331. Sodium citrate is employed as a flavoring agent in certain varieties of club soda. Sodium citrate is common as an ingredient in Bratwurst, and is also used in commercial ready to drink beverages and drink mixes, contributing a tart flavor. It is found in ice-cream, jams, sweets, milk powder, processed cheeses, carbonated beverages, and wine.
Buffer
As a conjugate base of a weak acid, citrate can perform as a buffering agent or acidity regulator, resisting changes in pH. Sodium citrate is used to control acidity in some substances, such as gelatin desserts. It can be found in the mini milk containers used with coffee machines. The compound is the product of antacids, such as Alka-Seltzer, when they are dissolved in water.
Medical uses
In 1914, the Belgian doctor Albert Hustin and the Argentine physician and researcher Luis Agote successfully used sodium citrate as an anticoagulant in blood transfusions. It continues to be used today in blood collection tubes and for the preservation of blood in blood banks. The citrate ion chelates calcium ions in the blood by forming calcium citrate complexes, disrupting the blood clotting mechanism.
In 2003, Oöpik, et al., showed the use of sodium citrate (0.5 grams per kg of body weight) improved running performance over 5 km by 30 seconds.[3]
Sodium citrate is used to relieve discomfort in urinary tract infections, such as cystitis, to reduce the acidosis seen in distal renal tubular acidosis, and can also be used as an osmotic laxative. It is a major component of the WHO Oral Rehydration Solution.
It is used as an antacid, especially prior to anaesthesia, for caesarian section procedures to reduce the risks associated with the aspiration of gastric contents.
Boiler descaling
Sodium citrate is a particularly effective agent for removal of carbonate scale from boilers without removing them from operation[4] and for cleaning automobile radiators.[5]
See also
References
- ^ "CRC Handbook of Chemistry and Physics". Retrieved 22 November 2013.
- ^ http://chem.sis.nlm.nih.gov/chemidplus/rn/68-04-2
- ^ V Oöpik, I Saaremets, L Medijainen, K Karelson, T Janson, S Timpmann (2003). "Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners". Br J Sports Med. 37 (6): 485–489. doi:10.1136/bjsm.37.6.485. PMC 1724692. PMID 14665584.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ U.S. patent 3,095,862
- ^ "MSDS" (PDF).

