Cyclopentadiene: Difference between revisions
Updating {{chembox}} (no changed fields - updated 'UNII_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemica |
Cp cmpxs are of not commercial, I dont think, maybe ferrocene in gasoline, probably should shift organometallic content to metallocene, rhodocene again? |
||
| Line 50: | Line 50: | ||
'''Cyclopentadiene''' is an [[organic compound]] with the [[Chemical formula|formula]] C<sub>5</sub>H<sub>6</sub>. This colorless liquid has a strong and [[unpleasant odor]]. At room temperature, this cyclic [[diene]] [[Dimer (chemistry)|dimer]]izes over the course of hours to give [[dicyclopentadiene]] via a [[Diels-Alder reaction]]. This dimer "cracks" to give the monomer upon heating. |
'''Cyclopentadiene''' is an [[organic compound]] with the [[Chemical formula|formula]] C<sub>5</sub>H<sub>6</sub>. This colorless liquid has a strong and [[unpleasant odor]]. At room temperature, this cyclic [[diene]] [[Dimer (chemistry)|dimer]]izes over the course of hours to give [[dicyclopentadiene]] via a [[Diels-Alder reaction]]. This dimer "cracks" to give the monomer upon heating. |
||
The compound is the |
The compound is mainly used for the production of cyclopentene and its derivatives. In the teaching laboratories, it is popularly used as a precursor to cyclopentadienyl-[[ligand]] ('Cp') in [[cyclopentadienyl complex]]es in [[organometallic chemistry]].<ref>Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X.</ref> Both cyclopentadiene and dicyclopentadiene can serve as ligands as well, but such complexes are uncommon. |
||
==Production and reactions== |
==Production and reactions== |
||
| Line 80: | Line 80: | ||
===Metallocene derivatives=== |
===Metallocene derivatives=== |
||
[[Image:Protonated rhodocene.svg|thumb|left|[(η<sup>5</sup>-C<sub>5</sub>H<sub>5</sub>)Rh(η<sup>4</sup>-C<sub>5</sub>H<sub>6</sub>)], an 18-valence electron mixed hapticity [[rhodocene]] derivative<ref>{{cite journal|title = Uber Aromatenkomplexe von Metallen: '''''LXXXVIII.''''' Uber Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Uber Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe|year = 1966|last1 = Fischer|first1 = E. O.|last2 = Wawersik|first2 = H.|authorlink1 = Ernst Otto Fischer|journal = [[Journal of Organometallic Chemistry|J. Organomet. Chem.]]|volume = 5|issue = 6|pages = 559–567|language = German|url = |doi = 10.1016/S0022-328X(00)85160-8}}</ref> that can form when the rhodocene monomer is |
[[Image:Protonated rhodocene.svg|thumb|left|[(η<sup>5</sup>-C<sub>5</sub>H<sub>5</sub>)Rh(η<sup>4</sup>-C<sub>5</sub>H<sub>6</sub>)], an 18-valence electron mixed hapticity [[rhodocene]] derivative<ref>{{cite journal|title = Uber Aromatenkomplexe von Metallen: '''''LXXXVIII.''''' Uber Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Uber Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe|year = 1966|last1 = Fischer|first1 = E. O.|last2 = Wawersik|first2 = H.|authorlink1 = Ernst Otto Fischer|journal = [[Journal of Organometallic Chemistry|J. Organomet. Chem.]]|volume = 5|issue = 6|pages = 559–567|language = German|url = |doi = 10.1016/S0022-328X(00)85160-8}}</ref> that can form when the rhodocene monomer is protonated.]] |
||
{{main|metallocene}} |
{{main|metallocene}} |
||
Most cyclopentadienyl compounds are instead [[organometallic chemistry|organometallic]] with metal-carbon bonding with a high degree of [[covalent bond|covalency]]. [[Deprotonation]] can be achieved with a variety of bases. The anion serves as a weak nucleophile in [[organic synthesis]] and combines with numerous anhydrous halides of the [[transition metal]]s to form [[cyclopentadienyl complex]]es, such as [[metallocene]]s.<ref>{{cite book |last1= Girolami|first1= G. S.|last2= Rauchfuss|first2= T. B.|last3= Angelici|first3= R. J.|title= Synthesis and Technique in Inorganic Chemistry|year= 1999|publisher= University Science Books|location= Mill Valley, CA|isbn= 0935702482}}</ref> As a simple example, [[ferrocene]] forms upon treating [[iron(II) chloride]] with a mixture of [[potassium hydroxide]] and cyclopentadiene in [[1,2-dimethoxyethane]].<ref>{{cite book |last1= Jolly|first1= W. L.|title= The Synthesis and Characterization of Inorganic Compounds|year= 1970|publisher= Prentice-Hall|location= Englewood Cliffs, NJ|isbn= 0138799326}}</ref> |
Although of little commercial value, metallocenes and related cyclopentadienyl derivatives have been heavily investigated. Most cyclopentadienyl compounds are instead [[organometallic chemistry|organometallic]] with metal-carbon bonding with a high degree of [[covalent bond|covalency]]. [[Deprotonation]] can be achieved with a variety of bases. The anion serves as a weak nucleophile in [[organic synthesis]] and combines with numerous anhydrous halides of the [[transition metal]]s to form [[cyclopentadienyl complex]]es, such as [[metallocene]]s.<ref>{{cite book |last1= Girolami|first1= G. S.|last2= Rauchfuss|first2= T. B.|last3= Angelici|first3= R. J.|title= Synthesis and Technique in Inorganic Chemistry|year= 1999|publisher= University Science Books|location= Mill Valley, CA|isbn= 0935702482}}</ref> As a simple example, [[ferrocene]] forms upon treating [[iron(II) chloride]] with a mixture of [[potassium hydroxide]] and cyclopentadiene in [[1,2-dimethoxyethane]].<ref>{{cite book |last1= Jolly|first1= W. L.|title= The Synthesis and Characterization of Inorganic Compounds|year= 1970|publisher= Prentice-Hall|location= Englewood Cliffs, NJ|isbn= 0138799326}}</ref> |
||
:FeCl<sub>2</sub>.4H<sub>2</sub>O + 2 C<sub>5</sub>H<sub>6</sub> + 2 KOH → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 2 KCl + 6 H<sub>2</sub>O |
:FeCl<sub>2</sub>.4H<sub>2</sub>O + 2 C<sub>5</sub>H<sub>6</sub> + 2 KOH → Fe(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub> + 2 KCl + 6 H<sub>2</sub>O |
||
| Line 89: | Line 89: | ||
==Uses== |
==Uses== |
||
Cyclopentadiene is a precursor to |
Cyclopentadiene is mainly useful as a precursor to [[cyclopentene]] and related monomers such as ethylidenenorbornene. Such species are used in the production of specialty polymers. |
||
==See also== |
==See also== |
||
Revision as of 15:52, 12 February 2011
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
pentole, pyropentylene, CPD, CpH
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.008.033 | ||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6 | |||
| Molar mass | 66.10 g/mol | ||
| Appearance | colourless liquid | ||
| Density | 0.786 g/cm³, liquid | ||
| Melting point | -85 °C (188 K) | ||
| Boiling point | 41 °C (314 K) | ||
| Insoluble | |||
| Acidity (pKa) | 16 | ||
| Structure | |||
| Planar[1] | |||
| Hazards | |||
| Flash point | 25 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels-Alder reaction. This dimer "cracks" to give the monomer upon heating.
The compound is mainly used for the production of cyclopentene and its derivatives. In the teaching laboratories, it is popularly used as a precursor to cyclopentadienyl-ligand ('Cp') in cyclopentadienyl complexes in organometallic chemistry.[2] Both cyclopentadiene and dicyclopentadiene can serve as ligands as well, but such complexes are uncommon.
Production and reactions
Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they are interconverted. They are obtained from coal tar (about 10 – 20 g/ton) and steam cracking from naphtha (about 14 kg/ton).[3]
Sigmatropic rearrangement
The hydrogen atoms in cyclopentadiene undergo rapid [1,5]-sigmatropic shifts as indicated by 1H NMR spectra recorded at various temperatures.[4]
Diels-Alder reactions
Famously, cyclopentadiene dimerizes via a reversible Diels-Alder reaction. The rate is convenient at room temperature, and the monomer can be stored for days at -20 °C.[3]
| Relative rate of dimerization of C5H6 | Temperature °C |
|---|---|
| 0.05 | _20 |
| 0.5 | 0 |
| 3.5 | 25 |
| 15 | 40 |
Cyclopentadiene readily undergoes other Diels-Alder reactions with dienophiles such as 1,4-benzoquinone.[5] Cycloaddition of O2 gives the bicyclic peroxide.
Deprotonation
The compound is unusually acidic (pKa 16) for a hydrocarbon, a fact explained by the high stability of the aromatic cyclopentadienyl anion, C5H5−. Simple compounds of this anion (such as the commercially available sodium cyclopentadienide) are often depicted as salts, although the free anion is not present in appreciable quantities in solution.[citation needed]
Metallocene derivatives
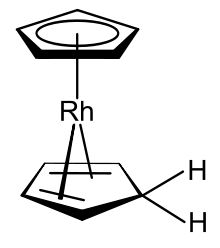
Although of little commercial value, metallocenes and related cyclopentadienyl derivatives have been heavily investigated. Most cyclopentadienyl compounds are instead organometallic with metal-carbon bonding with a high degree of covalency. Deprotonation can be achieved with a variety of bases. The anion serves as a weak nucleophile in organic synthesis and combines with numerous anhydrous halides of the transition metals to form cyclopentadienyl complexes, such as metallocenes.[7] As a simple example, ferrocene forms upon treating iron(II) chloride with a mixture of potassium hydroxide and cyclopentadiene in 1,2-dimethoxyethane.[8]
- FeCl2.4H2O + 2 C5H6 + 2 KOH → Fe(C5H5)2 + 2 KCl + 6 H2O
Organometallic complexes which include both the cyclopentadienyl anion and cyclopentadiene itself are known, one example of which is the rhodocene derivative produced from the rhodocene monomer in protic solvents.[9]
Uses
Cyclopentadiene is mainly useful as a precursor to cyclopentene and related monomers such as ethylidenenorbornene. Such species are used in the production of specialty polymers.
See also
- Aromaticity
- Methylcyclopentadiene and pentamethylcyclopentadiene, also found as ligands in organometallic chemistry
References
- ^ Valery I. Faustov, Mikhail P. Egorov, Oleg M. Nefedov and Yuri N. Molin (2000). "Ab initio G2 and DFT calculations on electron affinity of cyclopentadiene, silole, germole and their 2,3,4,5-tetraphenyl substituted analogs : structure, stability and EPR parameters of the radical anions". Phys. Chem. Chem. Phys. 2: 4293–4297. doi:10.1039/b005247g.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X.
- ^ a b Dieter Hönicke, Ringo Födisch, Peter Claus, Michael Olson “Cyclopentadiene and Cyclopentene” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_227
- ^ Streitwieser, A.; Heathcock, C. H.; Kosower, E. M. (1998). Introduction to Organic Chemistry (4th Edn.) Upper Saddle River, NJ: Prentice Hall.
- ^ Masaji Oda, Takeshi Kawase, Tomoaki Okada, and Tetsuya Enomoto (1998). "2-Cyclohexene-1,4-dione". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 186. - ^ Fischer, E. O.; Wawersik, H. (1966). "Uber Aromatenkomplexe von Metallen: LXXXVIII. Uber Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Uber Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe". J. Organomet. Chem. (in German). 5 (6): 559–567. doi:10.1016/S0022-328X(00)85160-8.
- ^ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 0935702482.
- ^ Jolly, W. L. (1970). The Synthesis and Characterization of Inorganic Compounds. Englewood Cliffs, NJ: Prentice-Hall. ISBN 0138799326.
- ^ Kolle, U.; Grub, J. (1985). "Permethylmetallocene 5 Reactions of Decamethylruthenium Cations". J. Organomet. Chem. 289 (1): 133–139. doi:10.1016/0022-328X(85)88034-7.


