Rutherford scattering experiments: Difference between revisions
m Fixed formatting of an "alpha particles" link |
GrahamBould (talk | contribs) mNo edit summary |
||
| Line 4: | Line 4: | ||
The '''Geiger-Marsden experiment''' (also called the '''Gold foil experiment''' or the '''Rutherford experiment''') was an experiment done by [[Hans Geiger]] and [[Ernest Marsden]] in [[1909]], under the direction of [[Ernest Rutherford]] at the Physical Laboratories of the [[University of Manchester]] which led to the downfall of the [[plum pudding model]] of the [[atom]]. |
The '''Geiger-Marsden experiment''' (also called the '''Gold foil experiment''' or the '''Rutherford experiment''') was an experiment done by [[Hans Geiger]] and [[Ernest Marsden]] in [[1909]], under the direction of [[Ernest Rutherford]] at the Physical Laboratories of the [[University of Manchester]] which led to the downfall of the [[plum pudding model]] of the [[atom]]. |
||
They measured the deflection of [[alpha particle]]s directed normally onto a sheet of very thin gold foil. Under the prevailing plum pudding model, the alpha particles should all have been deflected by at most |
They measured the deflection of [[alpha particle]]s directed normally onto a sheet of very thin gold foil. Under the prevailing plum pudding model, the alpha particles should all have been deflected by, at most, a few degrees. However they observed that a very small percentage of particles were deflected through angles much larger than 90 degrees. From this Rutherford concluded that the atom contained a very small positive charge which could repel the alpha particles if they came close enough, subsequently developed into the [[Bohr model]]. |
||
==Methodology== |
==Methodology== |
||
Revision as of 13:20, 24 April 2006
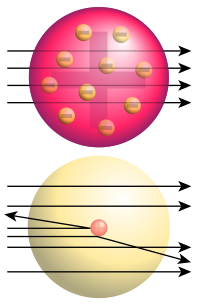
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge.
The Geiger-Marsden experiment (also called the Gold foil experiment or the Rutherford experiment) was an experiment done by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester which led to the downfall of the plum pudding model of the atom.
They measured the deflection of alpha particles directed normally onto a sheet of very thin gold foil. Under the prevailing plum pudding model, the alpha particles should all have been deflected by, at most, a few degrees. However they observed that a very small percentage of particles were deflected through angles much larger than 90 degrees. From this Rutherford concluded that the atom contained a very small positive charge which could repel the alpha particles if they came close enough, subsequently developed into the Bohr model.
Methodology
Geiger and Marsden bombarded a number of different metal foils with alpha particles generated from a tube of radium bromide gas. A low power microscope was used to count the scattering of these particles, a procedure requiring many hours in a darkened room watching for tiny flashes of light as the scattered particles struck a zinc sulfide scintillant screen.
A variety of different foils were used such as aluminium, iron, gold and lead along with different thicknesses of gold foil made by packing several pieces of very thin foil together. Given the very high mass and momentum of an alpha particle, the expectation was that the particles would pass through having being deflected by a tiny angle at most, with the number of particles penetrating falling off as the thickness of foil (and the atomic weight of its material) was increased; the remainder being absorbed.
However they were astonished to find that although this was generally true, around 1 in 8000 particles were reflected through more than 90 degrees even with a single sheet of extremely thin, 6x10-8 metre (or about 200 atoms) thick, gold foil, an observation completely at odds with the predictions of the plum pudding model.
Conclusions
The result was completely unpredicted, prompting Rutherford to later comment "It was almost as incredible as if you fired a fifteen-inch shell at a piece of tissue paper and it came back and hit you".
Early in 1911 Rutherford published a revised model of the atom, known as the Rutherford atom. The observations indicated that a model of the atom with a diffuse positive charge was incorrect and that it was instead concentrated. He concluded that the atom is mostly empty space, with most of the atom's mass concentrated in a tiny center, the nucleus and electrons being held in orbit around it by electrostatic attraction. The nucleus was around 10-15 meters in diameter, in the centre of a 10-10 metre diameter atom. Those alpha particles that had come into close proximity with the nucleus had been strongly deflected whereas the majority had passed at a relatively great distance to it.
Rutherford's model was developed by Niels Bohr into the Bohr model proposed in 1913. The Rutherford atom had a number of problems, in particular electrons should radiate electromagnetic energy and rapidly spiral into the nucleus.
See also
References
- Geiger H. & Marsden E. (1909). "On a Diffuse Reflection of the α-Particles". Proceedings of the Royal Society, Series A. 82: 495–500.
- Rutherford E. (1911). "The Scattering of α and β Particles by Matter and the Structure of the Atom". Philosophical Magazine, Series 6. 21: 669–688.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - JPEG images of Rutherford's 1911 paper
- Description of the experiment, from the New Mexico Institute of Mining and Technology
- Short biography of Ernest Rutherford
