2C-B: Difference between revisions
this level of wikilinking is unecessary |
m Chembox: rm/replace deprecated params. Fix unknown parameters (via AWB script) |
||
| Line 2: | Line 2: | ||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = |
| verifiedrevid = 477316294 |
||
| Reference =<ref>[http://www.fermentek.co.il/alamethicin.htm Alamethicin product page] from [[Fermentek]]</ref> |
|||
| ImageFile1 = 2C-B-Chemdraw.png |
|||
| ImageFile =Alamethicin.png |
|||
| ImageSize1 = |
|||
| ImageSize =250px |
|||
| ImageFile2 = 2C-B-3d-sticks.png |
|||
| IUPACName =''N''-​acetyl-​2-​methylalanyl-​<small>L</small>-​prolyl-​2-​methylalanyl-​<small>L</small>-​alanyl-​2-​methylalanyl-​<small>L</small>-​alanyl-​<small>L</small>-​glutaminyl-​2-​methylalanyl-​<small>L</small>-​valyl-​2-​methylalanylglycyl-​<small>D</small>-​leucyl-​2-​methylalanyl-​<small>L</small>-​prolyl-​<small>L</small>-​valyl-​2-​methylalanyl-​2-​methylalanyl-​<small>L</small>-​α-​glutamyl-​''N''<sup>1</sup>-​[(1''S'')-​1-​benzyl-​2-​hydroxyethyl]-​<small>L</small>-​glutamamide |
|||
| ImageSize2 = |
|||
| OtherNames = |
|||
| ImageFile3 = 4-bromo-2,5-dimethoxyphenethylamine.jpg |
|||
|Section1={{Chembox Identifiers |
|||
| ImageSize3 = |
|||
| IUPACName = 2-(4-bromo-2,5-dimethoxyphenyl)ethanamine |
|||
| OtherNames = |
|||
| Section1 = {{Chembox Identifiers |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = |
| ChemSpiderID = 17288702 |
||
| InChI = 1/C92H150N22O25/c1-47(2)43-58(72(127)108-92(24,25)84(139)113-41-29-33-59(113)73(128)103-65(48(3)4)75(130)111-90(20,21)82(137)112-89(18,19)80(135)102-56(37-40-64(120)121)70(125)101-55(35-38-61(93)117)69(124)98-54(46-115)44-53-31-27-26-28-32-53)99-63(119)45-95-77(132)85(10,11)110-76(131)66(49(5)6)104-81(136)88(16,17)107-71(126)57(36-39-62(94)118)100-67(122)50(7)96-78(133)86(12,13)106-68(123)51(8)97-79(134)87(14,15)109-74(129)60-34-30-42-114(60)83(138)91(22,23)105-52(9)116/h26-28,31-32,47-51,54-60,65-66,115H,29-30,33-46H2,1-25H3,(H2,93,117)(H2,94,118)(H,95,132)(H,96,133)(H,97,134)(H,98,124)(H,99,119)(H,100,122)(H,101,125)(H,102,135)(H,103,128)(H,104,136)(H,105,116)(H,106,123)(H,107,126)(H,108,127)(H,109,129)(H,110,131)(H,111,130)(H,112,137)(H,120,121)/t50-,51-,54-,55-,56-,57-,58-,59-,60-,65-,66-/m0/s1 |
|||
| InChI = 1/C10H14BrNO2/c1-13-9-6-8(11)10(14-2)5-7(9)3-4-12/h5-6H,3-4,12H2,1-2H3 |
|||
| SMILES1 = CC(C)CC(C(=O)NC(C)(C)C(=O)N1CCCC1C(=O)NC(C(C)C)C(=O)NC(C)(C)C(=O)NC(C)(C)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)N)C(=O)NC(Cc2ccccc2)CO)NC(=O)CNC(=O)C(C)(C)NC(=O)C(C(C)C)NC(=O)C(C)(C)NC(=O)C(CCC(=O)N)NC(=O)C(C)NC(=O)C(C)(C)NC(=O)C(C)NC(=O)C(C)(C)NC(=O)C3CCCN3C(=O)C(C)(C)NC(=O)C |
|||
| InChIKey = YMHOBZXQZVXHBM-UHFFFAOYAK |
|||
| InChIKey = LGHSQOCGTJHDIL-UTXLBGCNBC |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| ChEMBL = |
| ChEMBL = 438243 |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C92H150N22O25/c1-47(2)43-58(72(127)108-92(24,25)84(139)113-41-29-33-59(113)73(128)103-65(48(3)4)75(130)111-90(20,21)82(137)112-89(18,19)80(135)102-56(37-40-64(120)121)70(125)101-55(35-38-61(93)117)69(124)98-54(46-115)44-53-31-27-26-28-32-53)99-63(119)45-95-77(132)85(10,11)110-76(131)66(49(5)6)104-81(136)88(16,17)107-71(126)57(36-39-62(94)118)100-67(122)50(7)96-78(133)86(12,13)106-68(123)51(8)97-79(134)87(14,15)109-74(129)60-34-30-42-114(60)83(138)91(22,23)105-52(9)116/h26-28,31-32,47-51,54-60,65-66,115H,29-30,33-46H2,1-25H3,(H2,93,117)(H2,94,118)(H,95,132)(H,96,133)(H,97,134)(H,98,124)(H,99,119)(H,100,122)(H,101,125)(H,102,135)(H,103,128)(H,104,136)(H,105,116)(H,106,123)(H,107,126)(H,108,127)(H,109,129)(H,110,131)(H,111,130)(H,112,137)(H,120,121)/t50-,51-,54-,55-,56-,57-,58-,59-,60-,65-,66-/m0/s1 |
|||
| StdInChI = 1S/C10H14BrNO2/c1-13-9-6-8(11)10(14-2)5-7(9)3-4-12/h5-6H,3-4,12H2,1-2H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = |
| StdInChIKey = LGHSQOCGTJHDIL-UTXLBGCNSA-N |
||
| CASNo_Ref = {{cascite|changed| |
| CASNo_Ref = {{cascite|changed|??}} |
||
| CASNo = |
| CASNo =27061-78-5 |
||
| PubChem = |
| PubChem =16132042 |
||
| SMILES =CC(C)C[C@@H](C(=O)NC(C)(C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NC(C)(C)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC2=CC=CC=C2)CO)NC(=O)CNC(=O)C(C)(C)NC(=O)[C@H](C(C)C)NC(=O)C(C)(C)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@@H]3CCCN3C(=O)C(C)(C)NC(=O)C |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
}} |
|||
| ChEBI = 189669 |
|||
|Section2={{Chembox Properties |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| Formula =C<sub>92</sub>H<sub>150</sub>N<sub>22</sub>O<sub>25</sub> |
|||
| DrugBank = DB01537 |
|||
| MolarMass =1964.31 g/mol |
|||
| SMILES = COC1=C(Br)C=C(OC)C(CCN)=C1 |
|||
| Appearance =Off white solid |
|||
| Density = |
|||
| MeltingPtC = 255 to 270 |
|||
| MeltingPt_notes = |
|||
| BoilingPt = |
|||
| Solubility =Insoluble |
|||
| SolubleOther = Soluble |
|||
| Solvent = [[Dimethyl sulfoxide|DMSO]], [[methanol]], [[ethanol]] |
|||
}} |
|||
|Section3={{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
}} |
|||
}} |
}} |
||
'''Alamethicin''' is a [[peptide]] [[antibiotic]], produced by the fungus ''[[Trichoderma viride]]''. It belongs to [[peptaibol]] peptides which contain the [[non-proteinogenic]] [[amino acid residue]] Aib ([[2-Aminoisobutyric acid|2-aminoisobutyric acid]]). This residue strongly induces formation of [[alpha-helical]] structure. The peptide sequence is: |
|||
| Section2 = {{Chembox Properties |
|||
| Formula = C<sub>10</sub>H<sub>14</sub>BrNO<sub>2</sub> |
|||
| MolarMass = 260.13 g/mol |
|||
| Appearance = |
|||
| Density = |
|||
| MeltingPtC = |
|||
| BoilingPt = |
|||
| Solubility = }} |
|||
| Section3 = {{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = }} |
|||
}} |
|||
'''2C-B''' or '''2,5-dimethoxy-4-bromo[[phenethylamine]]''' is a [[psychedelic drug]] of the [[2C's|2C family]]. It was first synthesized by [[Alexander Shulgin]] in 1974. In Shulgin's book ''[[PiHKAL]]'', the dosage range is listed as 12–24 mg. 2C-B is sold as a white powder (sometimes as pink powder<ref>http://colombiareports.co/designer-drug-surfacing-colombias-club-scene/</ref>) sometimes pressed in tablets or gel caps and is referred to on the street by a number of slang names.<ref name="DEA - Evaluation Section">{{cite web |url=http://www.deadiversion.usdoj.gov/drugs_concern/bromo_dmp/bromo_dmp.pdf |title= 2C-B Street Names |date=2011-02-01 |accessdate= 2012-09-28|format=PDF| archiveurl= | archivedate= <!--DASHBot-->| deadurl= yes}}{{Dead link|date=July 2014}}</ref> The drug is usually taken orally, but can also be [[Insufflation (medicine)|insufflated]] or vaporized. |
|||
{{TOC limit|3}} |
|||
== History == |
|||
2C-B was synthesized from [[2,5-Dimethoxybenzaldehyde|2,5-dimethoxybenzaldehyde]] by [[Alexander Shulgin]] in 1974. It first saw use among the psychiatric community as an aid during therapy. It was considered one of the best drugs for this purpose because of its short duration, relative absence of side effects, and comparably mild nature.<ref name='erowid effects'/> Shortly after becoming popular in the medical community, it became popular recreationally. 2C-B was first sold commercially as an [[aphrodisiac]]<ref name="Reappears"/> under the trade name "Eros", which was manufactured by the German pharmaceutical company [[Drittewelle]].<ref name='dritte pack'>{{cite web|title=Drittewelle 2C-B Packaging|url=https://www.erowid.org/chemicals/show_image.php?i=2cb/2cb_pack2.jpg|publisher=Erowid.org|accessdate=25 September 2013|year=2002}}</ref> For several years, it was available as tablets in Dutch [[smart shop]]s under the name "Nexus". |
|||
Internationally, 2C-B is a [[Schedule II drug]] under the [[Convention on Psychotropic Substances]].<ref>{{cite web |url=http://www.incb.org/pdf/e/list/green.pdf |title= List of psychotropic substances under international control |accessdate= 2007-03-30|format=PDF| archiveurl= http://web.archive.org/web/20070302130637/http://www.incb.org/pdf/e/list/green.pdf| archivedate= 2 March 2007 <!--DASHBot-->| deadurl= no}}</ref> In the Netherlands, 2C-B became a list I substance of the Opium Law despite no health incidents occurring. Following the ban, other phenethylamines were sold in place of 2C-B until the Netherlands became the first country in the world to ban [[2C-I]], [[2C-T-2]] and [[2C-T-7]] alongside 2C-B. |
|||
In the [[United States]], a notice of proposed rulemaking published on December 20, 1994 in the ''[[Federal Register]]'' and after a review of relevant data, the Deputy Administrator of the [[Drug Enforcement Administration]] (DEA) proposed to place 4-[[Bromine|bromo]]-[[2C-H|2,5-DMPEA]] into [[Schedule I (US)|Schedule I]], making 2C-B illegal in the United States.<ref name='fedreg'>{{cite journal | title = Schedules of Controlled Substances; Proposed Placement of 4-bromo-2,5-dimethoxyphenethylamine into Schedule I | journal = Federal Register | date = 20 December 1994 | volume = 59 | issue = 243 | page = 65521| id = | url = http://isomerdesign.com/Cdsa/FR/59FR65521.pdf | format = PDF | accessdate = 2013-09-26}}</ref> This became permanent law on July 2, 1995.{{citation needed|date=September 2013}} |
|||
==Patterns of use== |
|||
2C-B first became popularized in the United States as a short-lived legal substitute for the street drug Ecstasy when [[MDMA]] became illegal in 1985.<ref name="Colombia" /> Many 2C-B users are young adults who attend [[rave]]s.<ref name="DEA - Evaluation Section" /> Though 2C-B is still used in the [[rave]] subculture, commonly mistaken for and/or sold as Ecstasy, its intentional use has become more common.<ref name="Gahlinger book">{{cite book|last=Gahlinger|first=Paul|title=Illegal Drugs: A Complete Guide to Their History, Chemistry, Use and Abuse|year=2004|publisher=Penguin|isbn=9780452285057|pages=343–344|url=http://books.google.com/books?id=kPszgXPVQAsC&pg=PT463&lpg=PT463&dqIqoAqKY6QGK4YDICg&ved=0CA0Q6AEwAQ#v=onepage&q&f=false}}</ref> In recent years, 2C-B has emerged as the drug of choice for club drug users in Colombia.<ref name=Colombia>{{cite news|last=Pachico|first=Elyssa|title='2CB Now Drug of Choice for Colombia Elite'|url=http://www.insightcrime.org/news-analysis/2c-b-now-drug-of-choice-for-colombia-elite|accessdate=11 February 2013|newspaper=InSight Crime|date=11/01/2012}}</ref> |
|||
Street prices range between $10 and $30 per tablet in the United States when bought in small quantities.<ref name="DEA - Evaluation Section" /> Larger retail purchases cost between $200 and $500 per gram. Wholesale purchases of 2C-B can lower the price to a range of $100 to $300 per gram.<ref name="Reappears">{{cite web|title=2C-B (Nexus) Reappears on the Club Drug Scene|url=http://www.justice.gov/archive/ndic/pubs0/665/665p.pdf|work=National Drug Intelligence Center|publisher=Department of Justice|accessdate=11 February 2013 |date=May 2001}}</ref> |
|||
==Toxicity and dosage== |
|||
The September 1998 ''Journal of Analytical Toxicology'' reported that very little data exists about the pharmacological properties, metabolism, and toxicity of 2C-B. The relationship between its use and death are unknown.<ref name="Reappears" /> The common oral recreational dose is around 15–25 mg,<ref name="urlErowid 2C-B Vault : Dose/Dosage">{{cite web |url=http://www.erowid.org/chemicals/2cb/2cb_dose.shtml |title=Erowid 2C-B Vault : Dose/Dosage }}</ref> at which visual and auditory effects are experienced. Severe adverse reactions are extremely rare, but use of 2C-B has been linked to significant injury in one case where the dosage is unknown, and the purity/identity of the chemical was not verified.<ref name="pmid20445431">{{cite journal |author=Ambrose JB, Bennett HD, Lee HS, Josephson SA |title=Cerebral vasculopathy after 4-bromo-2,5-dimethoxyphenethylamine ingestion |journal=The Neurologist |volume=16 |issue=3 |pages=199–202 |date=May 2010 |pmid=20445431 |doi=10.1097/NRL.0b013e3181a3cb53 }}</ref> |
|||
{| class="wikitable" |
|||
|- |
|||
!| |
|||
! width = "100px" | Oral |
|||
! width = "120px"| Insufflated |
|||
|- |
|||
| [[Effective dose (pharmacology)|ED<sub>50</sub>]] || 10 mg || 7–10 mg |
|||
|- |
|||
| Moderate || 15–20 mg || 13–19 mg |
|||
|- |
|||
| Strong || 21–35 mg || 20–30 mg |
|||
|- |
|||
| Extremely Intense || >35 mg || >31 mg |
|||
|- |
|||
| Duration || 2–7 hours || 2–5 hours |
|||
|} |
|||
The lethal dosage is unknown. It was reported in [[PiHKAL]], by [[Alexander Shulgin]], that a psychologist had accidentally taken a 100 mg dose orally without apparent harm.<ref name=pihkal>{{cite web|url=http://www.erowid.org/library/books_online/pihkal/pihkal020.shtml |title=Shulgin, A (1991) ''PIHKAL'' |publisher=Erowid.org |date= |accessdate=2012-05-15}}</ref> |
|||
When sold as "Ecstasy", tablets containing 2C-B often contain about 5 mg of the drug, an amount which produces stimulatory effects that mimic the effects of MDMA; in contrast, tablets marketed as 2C-B have larger quantities of the drug (10–20 mg) which cause hallucinogenic effects.<ref name="Netherlands 2c-b emergence" /> Street purity of 2C-B, when tested, has been found to be relatively high.<ref name="Review of public domain">{{cite journal |last=Cole |title=4-Bromo-2,5-dimethoxyphenethylamine (2C-B): a review of the public domain literature |journal=Science and Justice |date=October 2002 |volume=42 |issue=4 |pages=223–224 |doi=10.1016/S1355-0306(02)71832-7 |pmid=12632938 |url=http://lib.mdpu.org.ua/load/Chemistry/Organic%20chemistry/Organic%20syntheses/English%20edition/2c-b.review |accessdate=16 August 2012|display-authors=etal}}</ref> Researchers in Spain found that 2C-B samples in the country doubled between 2006 and 2009, switched from primarily powder form to tablets, and exhibited "low falsification rates".<ref name="Spain study">{{cite doi|10.1177/0269881111431752}}</ref> An analysis of street samples in the Netherlands found impurities "in small percentages"; only one of the impurities, the N-acetyl derivative of 2C-B, could be identified and comprised 1.3% of the sample. The authors suggested that this compound was a by-product of 2C-B synthesis.<ref name="Netherlands 2c-b emergence">{{cite doi |10.1093/jat/23.3.227}}</ref> |
|||
==Effects== |
|||
[[Image:2cb pill.jpg|thumb|right|2C-B pill with [[heart (symbol)|heart]] logo.]] |
|||
Little or no academic research has been conducted on the effects of 2C-B in humans. The information available is largely anecdotal and limited. Effects are often described as being more easily managed than other psychedelics;<ref name='erowid basics'>{{cite web | url = http://www.erowid.org/chemicals/2cb/2cb_basics.shtml | title = Erowid 2C-B Vault: Basics | accessdate = 2013-09-25 | date = 2011-02-20 | publisher = [[Erowid]]}}</ref> it is often compared to a mixture of a [[serotonergic psychedelic]] and [[MDMA]].<ref name="Spain study"/> |
|||
The effects of 2C-B include:<ref name='erowid effects'>{{cite web | url = http://www.erowid.org/chemicals/2cb/2cb_effects.shtml | title = Erowid 2C-B Vault: Effects | accessdate = 2013-09-25 | publisher = [[Erowid]]}}</ref><ref name='Drugscope'>{{cite web | url = http://www.drugscope.org.uk/resources/drugsearch/drugsearchpages | title = Drugscope: 2C-B | accessdate = 2013-09-25 | date = Jan 2004 | publisher = [[Drugscope]]}}</ref><ref name='dancesafe'>{{cite web | url = http://www.dancesafe.org/drug-information/2c-b | title = 2C-B - Dancesafe.org | accessdate = 2013-09-25 | publisher = [[Dancesafe]]}}</ref> |
|||
* At 5–10 mg, experiments with young chickens have shown it to produce effects similar to a low dosage of amphetamines.<ref>{{cite journal |author=Bronson ME, Jiang W, DeRuiter J, Clark CR |title=A behavioral comparison of Nexus, cathinone, BDB, and MDA |journal=Pharmacol. Biochem. Behav. |volume=51 |issue=2–3 |pages=473–5 |year=1995 |pmid=7667371 |doi=10.1016/0091-3057(95)00013-M }}</ref> At low doses the experience may shift in intensity from engaging to mild/undetectable. Experienced users report the ability to take control of the effects and switch from engaged to sober at will. |
|||
* The hallucinations have a tendency to decrease and then increase in intensity, giving the users a sense of “waves” or even glowing. These are popularly described as “clichéd ’70s visuals” or objects taking on "water color" like textures. |
|||
* While the effects of the drug often render users unable to concentrate deeply on anything in particular, some can become engrossed in an activity such as watching a movie or playing a video game, distracting themselves from the visual and auditory effects of the drug. |
|||
* Excessive giggling or smiling is common, as is a tendency for deeper “belly laughs”. |
|||
* Some users say that the effects are more intense when listening to music and report that they can see sounds and noises. |
|||
* Some users experience a decrease in visual acuity, although others report sharper vision. |
|||
* Increased awareness of one’s body; attention may be brought to perceived "imperfections" or internal body processes. |
|||
''The following effects are highly dose-dependent.'' |
|||
* Open eye visuals (OEVs), such as cartoon-like distortions and red or green halos around objects. Closed eye visuals (CEVs) are more common than OEVs. |
|||
* Affects and alters ability to communicate, engage in deep thought, or maintain attention span. |
|||
* Some users report experiencing frightening or fearful effects during the experience. Users describe feeling frigid or cold on reaching a plateau, while others feel wrapped in comfortable blankets/ultimate pleasure. |
|||
* Coordination may be affected, some users lose balance or have perceptual distinction problems. |
|||
Onset time of 2cb is highly dose dependent, but usually from 45 to 75 minutes. |
|||
===Use as an aphrodisiac=== |
|||
Before it was scheduled, 2C-B was sold in small doses as an aphrodisiac (see [[2C-B#History|History]]). Some users report [[aphrodisiac]] effects at lower doses (5–10 mg).<ref name=pihkal/><ref name='Drugscope'/> |
|||
===Side effects=== |
|||
* Some users report mild "jitters" (body tremors), shuddering breath, and/or mild muscle spasms after insufflating 2C-B. Whether or not these effects are enjoyable depends on the user. |
|||
* Mild to intense diarrhea, gas, nausea, and general gastrointestinal discomfort. |
|||
* Severe headaches after coming down from large doses have been reported. However, many users report a lack of "comedown" or "crash", instead noting a gradual return to sobriety. |
|||
* There are reports of hangover effects, especially when the drug is combined with alcohol. Some users have said to experience a slight irritability for roughly a day or so after use. However, these effects are rare and the drug is generally easier on the body than [[Methylenedioxymethamphetamine|MDMA]] (Ecstasy). |
|||
* At doses over 30–40 mg the user may experience frightening hallucinations, as well as [[tachycardia]], [[hypertension]], and [[hyperthermia]].<ref name=carmo>{{cite doi|10.1016/j.tox.2004.07.004}}</ref> |
|||
* 2C-B is very painful to insufflate. |
|||
===Duration=== |
|||
When orally consumed, 2C-B has a much longer delay before the onset of effects than when it is insufflated. Oral ingestion generally takes roughly 45–75 minutes for the effects to be felt, plateau lasts 2–4 hours, and coming down lasts 1–2 hours. Rectal administration ("plugged") onset varies from 5–20 minutes. Insufflated onset takes 1–10 minutes for effects to be felt. The duration can last from 4 to 12 hours depending on ROA, dose, and other factors.<ref name='erowid effects'>{{cite web | url = http://www.erowid.org/chemicals/2cb/2cb_effects.shtml | title Erowid 2C-B Vault: Effects | accessdate = 2014-04-10}}</ref> |
|||
With insufflation the effects are more abrupt and intense but have a significantly shorter duration, while oral usage results in a milder, longer experience. When insufflated, the onset happens very rapidly, usually reaching the peak at about 20–40 minutes and plateauing for 2–3 hours. 2C-B is also considered one of the most painful drugs to insufflate, with users reporting intense nasal burning<ref name='erowid basics'/> lasting as long as 30 minutes.<ref name='neurosoup'>{{cite web | url = http://www.neurosoup.com/2c-b/ | title = Neurosoup: 2C-B | accessdate = 2013-10-17 | last = Cole | first = Krystle | work = Neurosoup.com}}</ref> The sudden intensity of the experience combined with the pain can often start the experience with a negative imprint and nausea is also increased with insufflation, compounding the issue. |
|||
==Pharmacology== |
|||
Unlike most hallucinogens, 2C-B has been shown to be a low efficacy [[serotonin]] [[5-HT2A receptor|5-HT<sub>2A</sub> receptor]] [[partial agonist]]<ref name="moya 2007">{{cite journal |last1= Moya |first1= PR |title= Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors |journal= The Journal of Pharmacology and Experimental Therapeutics |volume= 321 |issue= 3 |pages= 1054–61 |year= 2007 |pmid= 17337633 |doi= 10.1124/jpet.106.117507 |display-authors=etal}}</ref> or even full antagonist.<ref>{{cite journal |author=Villalobos CA |title=4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes |journal=Br. J. Pharmacol. |volume=141 |issue=7 |pages=1167–74 |date=April 2004 |pmid=15006903 |pmc=1574890 |doi=10.1038/sj.bjp.0705722 |display-authors=etal}}</ref> This suggests that the [[5-HT2C receptor|5-HT<sub>2C</sub> receptor]] is primarily responsible for mediating the effects experienced by users of 2C-B, although functional antagonism of 5-HT<sub>2A</sub> or activation of the 5-HT<sub>2A</sub>-coupled [[phospholipase D]] pathway may also play a role.<ref name="moya 2007" /> The rank order of receptor [[antagonist]] potency for this family of drugs is [[2C-I]] > 2C-B > [[2C-D]] > [[2C-H]].{{citation needed|date=January 2013}}{{vague|date=January 2013}} |
|||
Research suggests that 2C-B increases [[dopamine]] levels in the brains of rats, which may contribute to its psychoactivity.<ref>{{cite doi|10.1007/s00213-012-2797-7}}</ref> |
|||
===Metabolism=== |
|||
2C-B has been shown to be metabolized by liver hepatocytes resulting in deamination and demethylation that produces several products. Oxidative deamination results in the 2-(4-bromo-2,5-dimethoxyphenyl)-ethanol (BDMPE) and 4-bromo-2,5-dimethoxyphenylacetic acid (BDMPAA) metabolites. Additionally, 4-bromo-2,5-dimethoxybenzoic acid (BDMBA) can be produced also by oxidative deamination. Further metabolism of BDMPE and BDMPAA may occur by demethylation. Alternatively, the later metabolites can be generated by demethylation of 2C-B followed by oxidative deamination.<ref name=carmo/> |
|||
There is species differentiation in the metabolism of 2C-B. Mice hepatocytes produce 4-bromo-2,5-dimethoxy-phenol (BDMP) a previously unknown metabolite. 2-(4-bromo-2-hydroxy-5-methoxyphenyl)-ethanol (B-2-HMPE) was produced by hepatocytes from human, monkey and rabbit but not by dog, rat and mouse.<ref name=carmo/> 2C-B also reduces aggressor responses in drugged rats.<ref>{{cite journal |author=Muehlenkamp F, Lucion A, Vogel WH |title=Effects of selective serotonergic agonists on aggressive behavior in rats |journal=Pharmacol. Biochem. Behav. |volume=50 |issue=4 |pages=671–4 |date=April 1995 |pmid=7617717 |doi=10.1016/0091-3057(95)00351-7 }}</ref> |
|||
===N-substituted derivatives=== |
|||
A variety of N-substituted derivatives of 2C-B have been tested, including N-methyl-2CB, N,N-dimethyl-2CB, N-ethyl-2CB and N-benzyl-2CB. Most simple alkyl derivatives were considerably less potent than 2C-B, with N-ethyl-2CB for instance having around 40× lower affinity at the 5-HT<sub>2A</sub> receptor. The N-benzyl derivative however was found to have higher [[binding affinity]] than 2C-B itself, with N-(4-bromobenzyl)-2CB binding even more tightly again.<ref name="pmid8027974">{{cite journal |author=Glennon RA |title=Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines |journal=Journal of Medicinal Chemistry |volume=37 |issue=13 |pages=1929–35 |date=June 1994 |pmid=8027974 |doi= 10.1021/jm00039a004|url=|display-authors=etal}}</ref> This initial research did not include functional assays of activity, but later led to the development of potent substituted N-benzyl derivatives such as [[25B-NBOMe]].<ref>{{cite thesis |url=http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221 |first=Ralf |last=Heim |title=Synthese und Pharmakologie potenter 5-HT<sub>2A</sub>-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts |trans-title=Synthesis and pharmacology of potent 5-HT<sub>2A</sub> receptor agonists which have a partial N-2-methoxybenzyl structure: Development of a new structure-activity concept |type= |language=German |publisher=[[Free University of Berlin]] |date=March 19, 2004 |accessdate=August 1, 2014}}</ref> |
|||
== Entheogenic use == |
|||
2C-B is used as [[entheogen]] by the [[Sangoma]], [[Nyanga people|Nyanga]], and [[Xhosa people#Folklore and religion|Amagqirha]] people over their traditional plants; they refer to the chemical as ''Ubulawu Nomathotholo'', which roughly translates to "''Medicine of the Singing Ancestors''".<ref>{{cite web|url=http://www.tacethno.com/info/2cb/2cbhistory.html#South%20Africa |title=2CB chosen over traditional entheogen's by South African healers. |publisher=Tacethno.com |date=2008-03-27 |accessdate=2012-05-15}}</ref><ref>[http://www.erowid.org/chemicals/2cb/2cb_article1.shtml The Nexus Factor - An Introduction to 2C-B] Erowid</ref><ref>[http://www.erowid.org/chemicals/show_image.php?i=2cb/ubulawu_pack.jpg Ubulawu Nomathotholo Pack] Photo by Erowid. © 2002 Erowid.org</ref> |
|||
==Drug prohibition laws== |
|||
===United Nations=== |
|||
The [[United Nations|UN]] [[Commission on Narcotic Drugs]] added 2C-B to Schedule II of the [[Convention on Psychotropic Substances]] in March 2001. LSD, psilocybin, and mescaline are in the more restrictive Schedule I. |
|||
2C-B is scheduled as a drug in most jurisdictions.<ref>{{cite web| url=http://www.erowid.org/chemicals/2cb/2cb_law.shtml |title=Erowid 2C-B page}}</ref> The following is a partial list of territories where the substance has been scheduled. |
|||
===Countries=== |
|||
====Argentina==== |
|||
It is controlled under the List 1, as well as other substances like [[2C-I]] or [[2C-T-2]].<ref>{{cite web|url=http://www.mpf.gov.ar/biblioteca/newsletter/n197/DECRETO_299_2010.pdf |title=Last Argentina Controlled Drugs List |format=PDF |accessdate=2012-05-15}}</ref> |
|||
====Australia==== |
|||
Controlled and on the list of substances subject to import and export controls (Appendix B:). Placed on Schedule One of the Drugs Misuse and Trafficking Act when it first came to notice in 1994, when in a showcase legal battle chemist R.Simpson was charged with manufacturing the substance in Sydney NSW. Alexander Shulgin came to Australia to testify on behalf of the defense (to no avail). |
|||
====Brazil==== |
|||
Controlled substance, making production, distribution, or possession illegal. |
|||
====Canada==== |
|||
CDSA Schedule III as "4-Bromo-2,5-dimethoxybenzeneethanamine and any salt, isomer or salt of isomer thereof".<ref>{{cite web |url=http://isomerdesign.com/Cdsa/schedule.php?schedule=3§ion=ALL&structure=C |title=CDSA Schedule II}}</ref> |
|||
====Denmark==== |
|||
2C-B is listed as a category B drug.<ref name='danish order on drugs'>{{cite web | url = https://www.retsinformation.dk/Forms/R0710.aspx?id=137169 | title = Bekendtgørelse om euforiserende stoffer | accessdate = 2013-10-01 | date = 2008-07-01 | language = Danish}}</ref> |
|||
====Estonia==== |
|||
Schedule I. |
|||
====Germany==== |
|||
Controlled in the Betäubungsmittelgesetz (BtMG) Anlage I as "Bromdimethoxyphenethylamin" (BDMPEA). |
|||
====Italy==== |
|||
2C-B is schedule I (tabella I)<ref>{{cite web |url=http://www.salute.gov.it/medicinaliSostanze/paginaInternaMedicinaliSostanze.jsp?id=7&menu=strumenti |title=Italy Drug Schedule (Tabella I)}}</ref> |
|||
====Japan==== |
|||
Scheduled Summer 1998. Previously marketed as "Performax". |
|||
====Netherlands==== |
|||
Scheduled on July 9, 1997. |
|||
====Norway==== |
|||
Schedule II as of March 22, 2004. Listed as 4-bromo-2,5-dimethoxyphenethylamine.<ref>{{cite web |url=http://www.lovdata.no/for/sf/ho/xo-19780630-0008.html |title=Norway Drug Schedule}}</ref> |
|||
====Poland==== |
|||
2C-B is schedule I (I-P group) in Poland. |
|||
====Spain==== |
|||
Added to Category 2 prohibited substances in 2002. |
|||
====Sweden==== |
|||
Schedule I in Sweden on Jun 1, 2002. 2C-B was first classified as "health hazard" under the act [[:sv:Lagen om förbud mot vissa hälsofarliga varor|''Lagen om förbud mot vissa hälsofarliga varor'']] (translated ''Act on the Prohibition of Certain Goods Dangerous to Health'') as of April 1, 1999 under '''SFS 1999:58'''<ref name='Sweden 1999:58'>{{cite web | url = http://www.notisum.se/rnp/sls/fakta/a9990058.HTM | title = Förordning (1999:58) om förbud mot vissa hälsofarliga varo | accessdate = 2013-10-01 | date = 1999-02-25 | language = Swedish}}</ref> that made it illegal to sell or possess. Then it became schedule I as of June 1, 2002, publicated in '''LVFS 2002:4'''<ref>http://www.lakemedelsverket.se/upload/lvfs/LVFS_2002-4.pdf</ref> but mislabeled "2-CB" in the document. However, this was corrected in a new document, '''LVFS 2009:22'''<ref>http://www.lakemedelsverket.se/upload/lvfs/LVFS_2009-22.pdf</ref> effective December 9, 2009. |
|||
Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phl |
|||
====Switzerland==== |
|||
Listed in Anhang D of the DetMV and is illegal to possess.<ref>{{cite web |url=http://www.swissmedic.ch/produktbereiche/00447/00536/index.html?lang=en&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1ad1IZn4Z2qZpnO2Yuq2Z6gpJCDdn12hGym162epYbg2c_JjKbNoKSn6A--&.pdf |title=Verzeichnis aller betäubungsmittelhaltigen Stoffe|format=PDF|accessdate = 2013-11-30|website = Swissmedic|publisher = Swiss Agency for Therapeutic Products|language = German|date = 2011-08-18|page = 2|trans_title = Directory of all narcotics-containing substances}}</ref> |
|||
(Ac = acetyl, Phl = [[phenylalaninol]], Aib = [[2-Aminoisobutyric acid]]) |
|||
====UK==== |
|||
All drugs in the 2C family are Class A under the 1971 Misuse of Drugs Act which means they are illegal to produce, supply or possess. Possession carries a maximum sentence of seven years imprisonment while supply is punishable by life imprisonment and an unlimited fine.<ref name='bbc'>{{cite web | url = http://www.bbc.co.uk/radio1/advice/factfile_az/2cb | title = BBC - Advice - 2CB | accessdate = 2013-10-01 | publisher = [[BBC]]}}</ref> |
|||
In [[cell membrane]]s, it forms [[voltage]]-dependent [[ion]] channels by aggregation of four to six [[molecules]]. |
|||
====USA==== |
|||
CSA Schedule I Section (d) Subsection (3) 4-Bromo-2,5-dimethoxyphenethylamine. |
|||
== |
== Biosynthesis == |
||
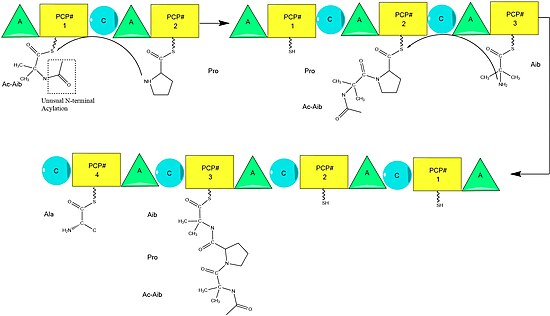
Alamethicin biosynthesis is hypothesized to be catalyzed by alamethicin synthase, a [[Nonribosomal peptide]] synthase (NRPS) first isolated in 1975.<ref>{{Cite journal| doi = 10.1016/0014-5793(76)80074-9| issn = 0014-5793| volume = 62| issue = 3| pages = 276–280| last1 = Rindfleisch| first1 = H.| last2 = Kleinkauf| first2 = H.| title = Biosynthesis of alamethicin| journal = FEBS Letters| accessdate = 2015-06-09| date = 1976-03-01| url = http://www.sciencedirect.com/science/article/pii/0014579376800749}}</ref> Although there are several sequences of the alamethicin peptide accepted,<ref>{{Cite journal| doi = 10.1002/psc.535| issn = 1075-2617| volume = 9| issue = 11-12| pages = 799–809| last1 = Kirschbaum| first1 = Jochen| last2 = Krause| first2 = Corina| last3 = Winzheimer| first3 = Ruth K.| last4 = Brückner| first4 = Hans| title = Sequences of alamethicins F30 and F50 reconsidered and reconciled| journal = Journal of Peptide Science: An Official Publication of the European Peptide Society| date = 2003-12| pmid = 14658799}}</ref> evidence suggests these all follow the general NRPS mechanism <ref>{{Cite journal| doi = 10.1021/cr960029e| issn = 0009-2665| volume = 97| issue = 7| pages = 2651–2674| last1 = Marahiel| first1 = Mohamed A.| last2 = Stachelhaus| first2 = Torsten| last3 = Mootz| first3 = Henning D.| title = Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis| journal = Chemical Reviews| accessdate = 2015-06-09| date = 1997-11-01| url = http://dx.doi.org/10.1021/cr960029e}}</ref> with small variations at select amino acids.<ref>{{Cite journal| issn = 0001-6187| volume = 22| issue = 4| pages = 411–418| last1 = Kleinkauf| first1 = H.| last2 = Rindfleisch| first2 = H.| title = Non-ribosomal biosynthesis of the cyclic octadecapeptide alamethicin| journal = Acta Microbiologica Academiae Scientiarum Hungaricae| date = 1975| pmid = 1241650}}</ref> Beginning with the [[acylation]] of the N terminal of the first [[2-Aminoisobutyric acid|aminoisobutiric acid]] on the ALM synthase enzyme by [[Acetyl-CoA]],<ref>{{Cite journal| issn = 0006-3002| volume = 526| issue = 2| pages = 375–386| last1 = Mohr| first1 = H.| last2 = Kleinkauf| first2 = H.| title = Alamethicin biosynthesis: acetylation of the amino terminus and attachment of phenylalaninol| journal = Biochimica Et Biophysica Acta| date = 1978-10-12| pmid = 568941}}</ref> this is followed by the sequential condensation of amino acids by each modular unit of the synthetase.<ref>{{Cite journal| doi = 10.1016/S0969-2126(00)00560-8| issn = 0969-2126| volume = 9| issue = 1| pages = –3-R9| last1 = Weber| first1 = Thomas| last2 = Marahiel| first2 = Mohamed A| title = Exploring the Domain Structure of Modular Nonribosomal Peptide Synthetases| journal = Structure| accessdate = 2015-06-09| date = 2001-01| url = http://www.sciencedirect.com/science/article/pii/S0969212600005608}}</ref> Amino acids are initially adenylated by an “[[adenylylation]]” (A) domain before being attached by a [[thioester]] bond to an [[Acyl Carrier Protein]]-like Peptidyl carrier protein.<ref name = Fischbach>{{Cite journal| doi = 10.1021/cr0503097| issn = 0009-2665| volume = 106| issue = 8| pages = 3468–3496| last1 = Fischbach| first1 = Michael A.| last2 = Walsh| first2 = Christopher T.| title = Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms| journal = Chemical Reviews| date = 2006-08| pmid = 16895337}}</ref> The growing chain is attached to the amino acid bearing PCP by the "condensation" (C) domain, followed by another round of the same reactions by the next module.<ref name = Fischbach /> |
|||
* [[Phenethylamine]] |
|||
[[File:NRPS basics corrected2.jpg|thumb|center|550px|alt=alt text|The general mechanism of NRPS synthesis in alamethicin, showing the condensation of amino acid substrates from module to module. Ac=Acetyl Aib=aminoisobutyric acid. Module components: A= Adenylylation PCP= Peptidyl Carrier Protein C=Condensation]] |
|||
* [[βk-2C-B]] |
|||
Assembly is completed by the addition of phenylalaninol, an unusual amino acid-like substrate.<ref>{{Cite journal| doi = 10.1021/ma00069a031| issn = 0024-9297| volume = 26| issue = 17| pages = 4617–4623| last1 = Turner| first1 = S. Richard| last2 = Voit| first2 = Brigitte I.| last3 = Mourey| first3 = Thomas H.| title = All-aromatic hyperbranched polyesters with C-phenylalaninol and N-acetate end groups: synthesis and characterization| journal = Macromolecules| accessdate = 2015-06-09| date = 1993-08-01| url = http://dx.doi.org/10.1021/ma00069a031}}</ref> Following addition of phenylalaninol the completed peptide chain is cleaved by the thioesterase domain, cleaving the thioester bond and leaving an alcohol. |
|||
* [[2C-B-FLY]] |
|||
[[File:Alamethicinbiosynth corrected2.jpg|thumb|center|550px|alt=alt text|A diagram of the individual modules and elongation of alamethicin biosynthesis. The growing peptide chain is shown for each module, ending in the clevage of the thioester and generation of linear alamethicin. Ac=Acetyl Aib=Aminoisobutyric acid Pheol=Phenylalaninol. Module components: A=Adenylylation PCP= Peptidyl Carrier Protein C=Condensation]] |
|||
* [[2,5-Dimethoxy-4-bromoamphetamine|DOB]] |
|||
* [[Bromo-DragonFLY]] |
|||
* [[BOB (psychedelic)]] |
|||
==References== |
== References == |
||
{{ |
{{reflist}} |
||
== |
== Further reading == |
||
*LR Jones, SW Maddock and HR Besch Jr. Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J. Biol. Chem., Vol. 255, Issue 20, 9971-9980, Oct, 1980. |
|||
* [http://www.erowid.org/library/books_online/pihkal/pihkal020.shtml 2C-B Entry in ''PiHKAL''] |
|||
* Explore structures of [http://www.ebi.ac.uk/pdbe/searchResults.html?display=both&term=NOR00010 Alamethicin] at the [[protein data bank]] |
|||
* [http://pihkal.info/read.php?domain=pk&id=20 2C-B Entry in PiHKAL • info] |
|||
* [http://bioinfo.lifl.fr/norine/result.jsp?ID=NOR00010 Alamethicin] in Norine |
|||
* [http://www.erowid.org/chemicals/2cb/2cb.shtml Erowid 2C-B vault]—includes reports from users of 2C-B, as well as scientific and government reports |
|||
** From "A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution." {{Cite pmid|6292726}} |
|||
* [http://www.erowid.org/chemicals/2cb/2cb_dose.shtml 2C-B Dosage chart] |
|||
* The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol <ref>{{Cite journal| doi = 10.1002/cbdv.200790095| issn = 1612-1880| volume = 4| issue = 6| pages = 1027–1051| last1 = Leitgeb| first1 = Balázs| last2 = Szekeres| first2 = András| last3 = Manczinger| first3 = László| last4 = Vágvölgyi| first4 = Csaba| last5 = Kredics| first5 = László| title = The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol| journal = Chemistry & Biodiversity| accessdate = 2015-06-09| date = 2007-06-01| url = http://onlinelibrary.wiley.com/doi/10.1002/cbdv.200790095/abstract}}</ref> |
|||
{{Non-ribosomally synthesized channels}} |
|||
{{Entactogens}} |
|||
{{Phenethylamines}} |
|||
{{Hallucinogenic phenethylamines}} |
|||
{{PiHKAL}} |
|||
{{Serotonergics}} |
|||
[[Category:Polypeptide antibiotics]] |
|||
{{DEFAULTSORT:2c-B}} |
|||
[[Category: |
[[Category:Antimicrobial peptides]] |
||
[[Category:Entactogens and empathogens]] |
|||
[[Category:Entheogens]] |
|||
[[Category:Bromoarenes]] |
|||
[[Category:Phenol ethers]] |
|||
Revision as of 21:14, 14 July 2015

| |
| Names | |
|---|---|
| IUPAC name
N-acetyl-2-methylalanyl-L-prolyl-2-methylalanyl-L-alanyl-2-methylalanyl-L-alanyl-L-glutaminyl-2-methylalanyl-L-valyl-2-methylalanylglycyl-D-leucyl-2-methylalanyl-L-prolyl-L-valyl-2-methylalanyl-2-methylalanyl-L-α-glutamyl-N1-[(1S)-1-benzyl-2-hydroxyethyl]-L-glutamamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.088 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C92H150N22O25 | |
| Molar mass | 1964.31 g/mol |
| Appearance | Off white solid |
| Melting point | 255 to 270 °C (491 to 518 °F; 528 to 543 K) |
| Insoluble | |
| Solubility in DMSO, methanol, ethanol | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alamethicin is a peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib (2-aminoisobutyric acid). This residue strongly induces formation of alpha-helical structure. The peptide sequence is:
Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phl
(Ac = acetyl, Phl = phenylalaninol, Aib = 2-Aminoisobutyric acid)
In cell membranes, it forms voltage-dependent ion channels by aggregation of four to six molecules.
Biosynthesis
Alamethicin biosynthesis is hypothesized to be catalyzed by alamethicin synthase, a Nonribosomal peptide synthase (NRPS) first isolated in 1975.[2] Although there are several sequences of the alamethicin peptide accepted,[3] evidence suggests these all follow the general NRPS mechanism [4] with small variations at select amino acids.[5] Beginning with the acylation of the N terminal of the first aminoisobutiric acid on the ALM synthase enzyme by Acetyl-CoA,[6] this is followed by the sequential condensation of amino acids by each modular unit of the synthetase.[7] Amino acids are initially adenylated by an “adenylylation” (A) domain before being attached by a thioester bond to an Acyl Carrier Protein-like Peptidyl carrier protein.[8] The growing chain is attached to the amino acid bearing PCP by the "condensation" (C) domain, followed by another round of the same reactions by the next module.[8]
Assembly is completed by the addition of phenylalaninol, an unusual amino acid-like substrate.[9] Following addition of phenylalaninol the completed peptide chain is cleaved by the thioesterase domain, cleaving the thioester bond and leaving an alcohol.

References
- ^ Alamethicin product page from Fermentek
- ^ Rindfleisch, H.; Kleinkauf, H. (1976-03-01). "Biosynthesis of alamethicin". FEBS Letters. 62 (3): 276–280. doi:10.1016/0014-5793(76)80074-9. ISSN 0014-5793. Retrieved 2015-06-09.
- ^ Kirschbaum, Jochen; Krause, Corina; Winzheimer, Ruth K.; Brückner, Hans (2003-12). "Sequences of alamethicins F30 and F50 reconsidered and reconciled". Journal of Peptide Science: An Official Publication of the European Peptide Society. 9 (11–12): 799–809. doi:10.1002/psc.535. ISSN 1075-2617. PMID 14658799.
{{cite journal}}: Check date values in:|date=(help) - ^ Marahiel, Mohamed A.; Stachelhaus, Torsten; Mootz, Henning D. (1997-11-01). "Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis". Chemical Reviews. 97 (7): 2651–2674. doi:10.1021/cr960029e. ISSN 0009-2665. Retrieved 2015-06-09.
- ^ Kleinkauf, H.; Rindfleisch, H. (1975). "Non-ribosomal biosynthesis of the cyclic octadecapeptide alamethicin". Acta Microbiologica Academiae Scientiarum Hungaricae. 22 (4): 411–418. ISSN 0001-6187. PMID 1241650.
- ^ Mohr, H.; Kleinkauf, H. (1978-10-12). "Alamethicin biosynthesis: acetylation of the amino terminus and attachment of phenylalaninol". Biochimica Et Biophysica Acta. 526 (2): 375–386. ISSN 0006-3002. PMID 568941.
- ^ Weber, Thomas; Marahiel, Mohamed A (2001-01). "Exploring the Domain Structure of Modular Nonribosomal Peptide Synthetases". Structure. 9 (1): –3-R9. doi:10.1016/S0969-2126(00)00560-8. ISSN 0969-2126. Retrieved 2015-06-09.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Fischbach, Michael A.; Walsh, Christopher T. (2006-08). "Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms". Chemical Reviews. 106 (8): 3468–3496. doi:10.1021/cr0503097. ISSN 0009-2665. PMID 16895337.
{{cite journal}}: Check date values in:|date=(help) - ^ Turner, S. Richard; Voit, Brigitte I.; Mourey, Thomas H. (1993-08-01). "All-aromatic hyperbranched polyesters with C-phenylalaninol and N-acetate end groups: synthesis and characterization". Macromolecules. 26 (17): 4617–4623. doi:10.1021/ma00069a031. ISSN 0024-9297. Retrieved 2015-06-09.
Further reading
- LR Jones, SW Maddock and HR Besch Jr. Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J. Biol. Chem., Vol. 255, Issue 20, 9971-9980, Oct, 1980.
- Explore structures of Alamethicin at the protein data bank
- Alamethicin in Norine
- From "A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution." Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 6292726, please use {{cite journal}} with
|pmid=6292726instead.
- From "A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution." Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 6292726, please use {{cite journal}} with
- The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol [1]
- ^ Leitgeb, Balázs; Szekeres, András; Manczinger, László; Vágvölgyi, Csaba; Kredics, László (2007-06-01). "The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol". Chemistry & Biodiversity. 4 (6): 1027–1051. doi:10.1002/cbdv.200790095. ISSN 1612-1880. Retrieved 2015-06-09.
