Bromobimane: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 38: | Line 38: | ||
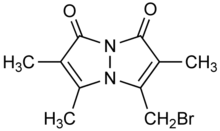
'''Bromobimane''' or '''monobromobimane''' is a [[heterocyclic compound]] and [[bimane dye]] that is used as a reagent in [[biochemistry]]. While bromobimane itself is essentially nonfluorescent, it [[alkylate]]s [[thiol]] groups, displacing the bromine and adding the fluorescent tag (λ<sub>emission</sub> = 478 nm) to the thiol. Its alkylating properties are comparable to [[iodoacetamide]].<ref>{{cite journal|author1=Paul C. Chinn|author2=Vincent Pigiet|author3=Robert C. Fahey|last-author-amp=yes|title=Determination of thiol proteins using monobromobimane labeling and high-performance liquid chromatographic analysis: Application to Escherichia coli thioredoxin|journal=Analytical Biochemistry|year=1986|volume=159|pages=143–149|doi=10.1016/0003-2697(86)90319-2|pmid=3544950|issue=1}}</ref> |
'''Bromobimane''' or '''monobromobimane''' is a [[heterocyclic compound]] and [[bimane dye]] that is used as a reagent in [[biochemistry]]. While bromobimane itself is essentially nonfluorescent, it [[alkylate]]s [[thiol]] groups, displacing the bromine and adding the fluorescent tag (λ<sub>emission</sub> = 478 nm) to the thiol. Its alkylating properties are comparable to [[iodoacetamide]].<ref>{{cite journal|author1=Paul C. Chinn|author2=Vincent Pigiet|author3=Robert C. Fahey|last-author-amp=yes|title=Determination of thiol proteins using monobromobimane labeling and high-performance liquid chromatographic analysis: Application to Escherichia coli thioredoxin|journal=Analytical Biochemistry|year=1986|volume=159|pages=143–149|doi=10.1016/0003-2697(86)90319-2|pmid=3544950|issue=1}}</ref> |
||
== In Vitro == |
|||
Bromobimanes in solution (aqueous or organic solvents of medium polarity) react with small thiols [e.g., the tripeptide thiol glutathione (GSH)], and with reactive protein thiol groups (e.g., hemoglobin). The reactions of bromobimanes with thiols are second order and dependent on pH, the active nucleophile being the thiolate anion. The reaction of bromobimane with a thiolate converts the nonfluorescent agent into water-soluble fluorescent products. The neutral agents mBBr and bBBr are moderately soluble in mediumpolarity organic solvents (acetonitrile, dichloromethane), and slightly soluble in water. The quaternary salt, qBBr, and the anionic bromobimane, SBBr, are soluble in water, but less soluble in organic solvents. Bromobimanes are yellow.<ref>{{Cite web|url=https://www.medchemexpress.com/Bromobimane.html|title=Bromobimane (Synonyms: Monobromobimane)|last=|first=|date=|website=MedChemExpress|archive-url=|archive-date=|dead-url=|access-date=}}</ref> |
|||
<br /> |
|||
==Synthesis== |
==Synthesis== |
||
Bromobimane is prepared from 3,4-dimethyl-2-pyrazolin-5-one (a condensation product of ethyl 2-methylacetoacetate with hydrazine) by chlorination followed by basic treatment; with aqueous K<sub>2</sub>CO<sub>3</sub> under heterogeneous conditions, the required ''syn''-bimane, 2,3,5,6-tetramethyl-1''H'',7''H''-pyrazolo[1,2-''a'']pyrazole-1,7-dione, is the major product. It can then be selectively brominated to the target bromobimane (with 1 equivalent of Br<sub>2</sub>; or dibromobimane, if 2 equivalents of Br<sub>2</sub> are used):<ref name=Bimanes_synthesis_JACS_1980>{{cite journal|author1=Kosower, Edward M.|author2=Pazhenchevsky, Barak|title=Bimanes. 5. Synthesis and Properties of ''syn''- and ''anti''-1,5-Diazabicyclo[3.3.0]octadienediones (9,10-Dioxabimanes)|journal=Journal of the American Chemical Society|volume=102|issue=15|pages=4983–4993|year=1980|doi=10.1021/ja00535a028}}</ref> |
Bromobimane is prepared from 3,4-dimethyl-2-pyrazolin-5-one (a condensation product of ethyl 2-methylacetoacetate with hydrazine) by chlorination followed by basic treatment; with aqueous K<sub>2</sub>CO<sub>3</sub> under heterogeneous conditions, the required ''syn''-bimane, 2,3,5,6-tetramethyl-1''H'',7''H''-pyrazolo[1,2-''a'']pyrazole-1,7-dione, is the major product. It can then be selectively brominated to the target bromobimane (with 1 equivalent of Br<sub>2</sub>; or dibromobimane, if 2 equivalents of Br<sub>2</sub> are used):<ref name=Bimanes_synthesis_JACS_1980>{{cite journal|author1=Kosower, Edward M.|author2=Pazhenchevsky, Barak|title=Bimanes. 5. Synthesis and Properties of ''syn''- and ''anti''-1,5-Diazabicyclo[3.3.0]octadienediones (9,10-Dioxabimanes)|journal=Journal of the American Chemical Society|volume=102|issue=15|pages=4983–4993|year=1980|doi=10.1021/ja00535a028}}</ref> |
||
Revision as of 07:08, 21 June 2019
| |
| |
| Names | |
|---|---|
| IUPAC name
3-(bromomethyl)-2,5,6-trimethyl-1H,7H-
| |
| Other names
Bromobimane, mBBr
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H11BrN2O2 | |
| Molar mass | 271.114 g·mol−1 |
| Melting point | 152 to 154 °C (306 to 309 °F; 425 to 427 K) |
| in MeOH, DMF, DMSO | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
alkylating agent |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bromobimane or monobromobimane is a heterocyclic compound and bimane dye that is used as a reagent in biochemistry. While bromobimane itself is essentially nonfluorescent, it alkylates thiol groups, displacing the bromine and adding the fluorescent tag (λemission = 478 nm) to the thiol. Its alkylating properties are comparable to iodoacetamide.[1]
In Vitro
Bromobimanes in solution (aqueous or organic solvents of medium polarity) react with small thiols [e.g., the tripeptide thiol glutathione (GSH)], and with reactive protein thiol groups (e.g., hemoglobin). The reactions of bromobimanes with thiols are second order and dependent on pH, the active nucleophile being the thiolate anion. The reaction of bromobimane with a thiolate converts the nonfluorescent agent into water-soluble fluorescent products. The neutral agents mBBr and bBBr are moderately soluble in mediumpolarity organic solvents (acetonitrile, dichloromethane), and slightly soluble in water. The quaternary salt, qBBr, and the anionic bromobimane, SBBr, are soluble in water, but less soluble in organic solvents. Bromobimanes are yellow.[2]
Synthesis
Bromobimane is prepared from 3,4-dimethyl-2-pyrazolin-5-one (a condensation product of ethyl 2-methylacetoacetate with hydrazine) by chlorination followed by basic treatment; with aqueous K2CO3 under heterogeneous conditions, the required syn-bimane, 2,3,5,6-tetramethyl-1H,7H-pyrazolo[1,2-a]pyrazole-1,7-dione, is the major product. It can then be selectively brominated to the target bromobimane (with 1 equivalent of Br2; or dibromobimane, if 2 equivalents of Br2 are used):[3]

Bromobimanes are light-sensitive compounds and should be kept refrigerated and protected from light.
References
- ^ Paul C. Chinn; Vincent Pigiet; Robert C. Fahey (1986). "Determination of thiol proteins using monobromobimane labeling and high-performance liquid chromatographic analysis: Application to Escherichia coli thioredoxin". Analytical Biochemistry. 159 (1): 143–149. doi:10.1016/0003-2697(86)90319-2. PMID 3544950.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ "Bromobimane (Synonyms: Monobromobimane)". MedChemExpress.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Kosower, Edward M.; Pazhenchevsky, Barak (1980). "Bimanes. 5. Synthesis and Properties of syn- and anti-1,5-Diazabicyclo[3.3.0]octadienediones (9,10-Dioxabimanes)". Journal of the American Chemical Society. 102 (15): 4983–4993. doi:10.1021/ja00535a028.
