Synthesis of precious metals: Difference between revisions
Jasper Deng (talk | contribs) →Cobalt: remove unsourced section with outlandish statements |
In fact revert back to edit before Hobbitschuster's contributions as everything added is unsourced. |
||
| Line 23: | Line 23: | ||
===Palladium=== |
===Palladium=== |
||
[[Palladium]] is also produced by nuclear fission in small percentages, amounting to 1 kg per ton of spent fuel. As opposed to rhodium and ruthenium, palladium has a radioactive isotope, <sup>107</sup>Pd, with a very long half-life (6.5 million years), so palladium produced in this way has a very low radioactive intensity. Mixed in with the other isotopes of palladium recovered from the spent fuel, this gives a radioactive dose rate of 7.207×10<sup>−5</sup> [[Curie (unit)|Ci]], which is well below the safe level of 1×10<sup>−3</sup> Ci.{{clarify|date=January 2022}} Also, <sup>107</sup>Pd has a very low decay energy of only 33 keV, and so would be unlikely to pose a hazard even if pure. But, the other isotope of Pd, <sup>108</sup>Pd , is not radioactive and popular( 26,46%, under <sup>106</sup>Pd with 27,33%) |
[[Palladium]] is also produced by nuclear fission in small percentages, amounting to 1 kg per ton of spent fuel. As opposed to rhodium and ruthenium, palladium has a radioactive isotope, <sup>107</sup>Pd, with a very long half-life (6.5 million years), so palladium produced in this way has a very low radioactive intensity. Mixed in with the other isotopes of palladium recovered from the spent fuel, this gives a radioactive dose rate of 7.207×10<sup>−5</sup> [[Curie (unit)|Ci]], which is well below the safe level of 1×10<sup>−3</sup> Ci.{{clarify|date=January 2022}} Also, <sup>107</sup>Pd has a very low decay energy of only 33 keV, and so would be unlikely to pose a hazard even if pure. But, the other isotope of Pd, <sup>108</sup>Pd , is not radioactive and popular( 26,46%, under <sup>106</sup>Pd with 27,33%). |
||
===Silver=== |
===Silver=== |
||
[[Silver]] is produced as result of nuclear fission in small amounts (approximately 0.1%). The vast majority of produced Silver is Ag-109 which is stable, and Ag 111 which decays away very quickly to form Cd 111. The only radioactive isotopes with a significant half lives produced are the [[metastable]] Ag-110m (249.8 d) and Ag-108m (418 years), the former of which is produced via neutron capture from Ag-109), and the latter of which is only formed in trace quantities. After a short period in storage the produced Silver is almost entirely stable and safe to use. Because of the modest price of silver, extraction of silver alone from highly radioactive fission products would be uneconomical. When recovered with ruthenium, rhodium, and palladium (price of silver in 2011: about 880 €/kg; rhodium; and ruthenium: about 30,000 €/kg) the economics change substantially: silver becomes a byproduct of platinoid metal recovery from fission waste and the marginal cost of processing the byproduct could be competitive. |
[[Silver]] is produced as result of nuclear fission in small amounts (approximately 0.1%). The vast majority of produced Silver is Ag-109 which is stable, and Ag 111 which decays away very quickly to form Cd 111. The only radioactive isotopes with a significant half lives produced are the [[metastable]] Ag-110m (249.8 d) and Ag-108m (418 years), the former of which is produced via neutron capture from Ag-109), and the latter of which is only formed in trace quantities. After a short period in storage the produced Silver is almost entirely stable and safe to use. Because of the modest price of silver, extraction of silver alone from highly radioactive fission products would be uneconomical. When recovered with ruthenium, rhodium, and palladium (price of silver in 2011: about 880 €/kg; rhodium; and ruthenium: about 30,000 €/kg) the economics change substantially: silver becomes a byproduct of platinoid metal recovery from fission waste and the marginal cost of processing the byproduct could be competitive. |
||
==Issues with recovery from fission products== |
|||
While the mere ''content'' of some precious metals (or indeed other valuable substances such as [[Tritium]], [[Caesium-137]], [[Krypton-85]] or stable isotopes of [[Xenon]]) would probably allow their economic recovery - [[Platinum group metal]] ores of lower content of platinum group metals than their [[fission product yield]] are routinely exploited profitably - there are big hurdles to actually recovering usable material at scale from spent nuclear fuel. The issues fall into three broad categories: Regulatory, proliferation concerns and economics. |
|||
===Regulatory issues=== |
|||
In general the [[precautionary principle]] is applied to a strong degree in radiation protection. This means that doses received by radiation workers and the general public are to be kept [[ALARA]] - as low as reasonably achievable. For that purpose, several "layers" of security have been put in place. For example, every object - including such "pedestrian" articles as sanitary wipes or pencils - that has been in the "hot" (i.e. legally defined as radioactive) part of a nuclear facility, be it a reactor or a reprocessing plant, is ipso facto [[low level waste]], no matter the actual radiation content. There might be ways around this regulation and it is of no concern to the production of [[radionuclides]] for use in medicine or industry, whose radioactivity is indeed the ''point'' of their production, but to produce a product that is - in fact and in the eyes of the law - non-radioactive by extracting it from spent nuclear fuel in a [[nuclear reprocessing]] plant (which is of course also "hot" in the radiation protection sense) is something that few countries provide for in their regulatory environment surrounding the nuclear industry. |
|||
===Proliferation concerns=== |
|||
Nuclear non-proliferation relies on several layers of safeguards. While there is active debate in the field on the necessity or indeed sufficiency of most of those safeguards, for the most part the regulatory environment has settled on a few key points. Separation of [[plutonium]] - even if [[reactor grade plutonium]], which at the very least is ''less'' suitable for bomb-making than [[weapons grade plutonium]] - from all other products is generally seen as a proliferation risk. Even countries that routinely do large scale nuclear reprocessing using the [[PUREX]] process (whose main aim historically was the recovery of highly chemically pure plutonium) are undertaking efforts to reduce the need to separate uranium and plutonium. The United States explicitly abandoned civilian reprocessing in the 1970s on non-proliferation grounds and has since then exerted significant international pressure to limit the capabilities and spread of reprocessing. At the same time, research projects like the [[Integral Fast Reactor]] which used a new [[Electrometallurgy|Electrometallurgical]] process called [[pyroprocessing]] with claimed non-proliferation benefits (all [[Actinide]]s are recovered together, at no point does a separate plutonium-stream arise) have failed to produce large scale production plants for political and economical reasons. As such there is only slight incentive to improve upon the PUREX process and efforts to replace it with a process more amenable to fission product recovery have not reached any notable successes as of the early 2020s. |
|||
===Economical concerns=== |
|||
It should not be forgotten that [[spent nuclear fuel]] remains, above all else, [[uranium]]. Even in (as of yet hardly deployed) [[fast reactor]]s, the ingrowth of [[neutron poison]]s among the fission products would shut down the reaction long before all fissile material is consumed. As such, all reprocessing is concerned first and foremost with the recovery of uranium and other fissile or [[fertile material]]. While [[reprocessed uranium]] has been used in some nuclear power plants, there is a large "legacy" stockpile of it with no current use, which may become attractive if prices on the [[uranium market]] rise or if those countries that hold the stockpiles are cut off from international uranium supplies. Both [[depleted uranium]] (of which even greater unused stockpiles exist) and reprocessed uranium could be used as a [[breeding blanket]] in future fast reactors or in thermal [[subcritical reactor]]s in "breeding" mode. Reactors capable of using [[natural uranium]] as fuel can also make use of most grades of reprocessed uranium, except of course that derived from their own spent fuel. However, the main reason Uranium is recovered during reprocessing is so as to reduce the volume of the waste. |
|||
From the standpoint of fuel for new nuclear reactors, recovering 90-97% of the spent fuel (90% or more of the mass is Uranium, about 1% is Plutonium) is a very good yield. Many industrial recovery processes have far lower efficiencies. The current state of the art is to [[vitrification|vitrify]] the waste into a chemically inert [[ceramic]] or glass-like form. Recovery of anything from this form is extremely difficult and this is by design, as the material is designed to last for geologic timescales in a [[deep geological repository]]. The PUREX process is ill-suited for a "wait and see" approach towards residual products currently regarded as waste, as it uses aqueous solution and highly concentrated [[nitric acid]] which transforms almost all the elements in the fuel - whether harmless, radiotoxic or chemically toxic - into highly mobile compounds or ionic solutions and as storage as liquid (as was done at the military [[Hanford site]]) presents obvious pollution risks, the liquid residue is transformed into the inert matrix as quickly as feasible. |
|||
As the Uranium price has been depressed since about the 1970s when large uranium deposits were discovered (The [[Uranium price bubble of 2007]] while it ''did'' lead to new prospecting activity had little effect on utilities who have long-term contracts and large stockpiles of fuel), there is little economic incentive to do any reprocessing at all. As such the needed expertise and equipment ([[hot cell]]s, [[remote manipulator]]s) are expensive and hard to come by even before the aforementioned proliferation concerns are figured in. Unlike in mining, it is not possible economically or from the regulatory standpoint for a [[start-up company]] to put a new approach into practical operation easily. As such, the industry tends to be dominated by state-owned or quasi state-owned companies with a long term outlook and relatively conservative approaches. Owners of large PUREX facilities will try to avoid their facilities becoming [[stranded assets]]. |
|||
As such while there is already a low fraction of spent fuel that is reprocessed ''at all'' for the reasons outlined above, the entities involved in the reprocessing by and large see everything that isn't a fissile or fertile nuclide as "waste" to be gotten rid of and not as a possible [[joint product]] of their activity. This is even the case where "particularly undesirable" and "particularly desirable" (depending on where the product is at any given time) align most strikingly. Ruthenium, for example, is generally deemed one of the most "problematic" substances in PUREX as the [[ruthenate]] ion can clog machinery and as several ruthenium compounds are volatile and can escape to the atmosphere. On the other hand, ruthenium is a precious metal and its targeted extraction could bring benefits to "both sides of the ledger" - the undesirable properties no longer hamper the reprocessing and the desirable properties can be used in industry or other fields. However, as the [[sunk cost]] of the gigantic processing sites and equipment is considerable, there is no desire to switch to a different reprocessing scheme, even if it were advantageous overall and in the long run. |
|||
Finally, the "waste" from the spent fuel, which contains most of the [[minor actinides]] and virtually all of the fission products remains highly radiotoxic, chemically toxic, heat emitting and of concern at the very least for a "[[dirty bomb]]" if not some more "exotic" types of nuclear fission bomb relying on isotopes such as [[Americium-241]]. As such there is very little incentive on the part of governments, regulators and reprocessing companies to "privatize" this effluent stream for any company that would try to extract valuable materials from it. In turn most companies do not even consider entering this field due to the high investment required, the uncertainty (whether reprocessing will continue to exist, whether a [[nuclear phaseout]] happens etc) and the regulatory burden. |
|||
==Precious metals produced via irradiation== |
==Precious metals produced via irradiation== |
||
| Line 68: | Line 51: | ||
The cost of [[platinum]] as of October 2014 was $39,900 per kilogram, making it equally as expensive as [[rhodium]]. [[Iridium]], by contrast, has only about half the value of platinum ($18,000/kg). Iridium has two naturally occurring isotopes, <sup>191</sup>Ir and <sup>193</sup>Ir. Irradiation by slow neutrons would transmute these isotopes into <sup>192</sup>Ir and <sup>194</sup>Ir, with short half-lives of 73 days and 19 hours, respectively; the predominant decay pathway for both of these isotopes is beta-minus decay into <sup>192</sup>Pt and <sup>194</sup>Pt.<ref name="ir194">{{cite web|url=http://www.periodictable.com/Isotopes/077.194/index.dm.html|title=Iridium 194}}</ref><ref name="ir192">{{cite web|url=http://www.periodictable.com/Isotopes/077.192/index.dm.html|title=Iridium 192}}</ref> |
The cost of [[platinum]] as of October 2014 was $39,900 per kilogram, making it equally as expensive as [[rhodium]]. [[Iridium]], by contrast, has only about half the value of platinum ($18,000/kg). Iridium has two naturally occurring isotopes, <sup>191</sup>Ir and <sup>193</sup>Ir. Irradiation by slow neutrons would transmute these isotopes into <sup>192</sup>Ir and <sup>194</sup>Ir, with short half-lives of 73 days and 19 hours, respectively; the predominant decay pathway for both of these isotopes is beta-minus decay into <sup>192</sup>Pt and <sup>194</sup>Pt.<ref name="ir194">{{cite web|url=http://www.periodictable.com/Isotopes/077.194/index.dm.html|title=Iridium 194}}</ref><ref name="ir192">{{cite web|url=http://www.periodictable.com/Isotopes/077.192/index.dm.html|title=Iridium 192}}</ref> |
||
=== Cobalt === |
|||
[[Cobalt]], despite having 110 tons-production per year, is also manufactured from iron. Firstly, isotopes of iron by slow neutrons would transmute these isotopes into <sup>59</sup>Fe, which has a half-life is 44.6 days. Its decay pathway for this isotope is beta-minus decay into <sup>59</sup>Co. Although this method is not too efficient, it provides the method to ensure that the wars and conflicts in Africa would stop in the near future. |
|||
===Gold=== |
===Gold=== |
||
Revision as of 17:29, 22 February 2022
This article possibly contains original research. (June 2016) |
This article needs additional citations for verification. (June 2016) |
The synthesis of precious metals involves the use of either nuclear reactors or particle accelerators to produce these elements.
Precious metals occurring as fission products
Ruthenium, rhodium
Ruthenium and rhodium are precious metals produced as a small percentage of the fission products from the nuclear fission of uranium. The longest half-lives of the radioisotopes of these elements generated by nuclear fission are 373.59 days for ruthenium and 45 days for rhodium[clarification needed]. This makes the extraction of the non-radioactive isotope from spent nuclear fuel possible after a few years of storage, although the extract must be checked for radioactivity from trace quantities of other elements before use.[1]
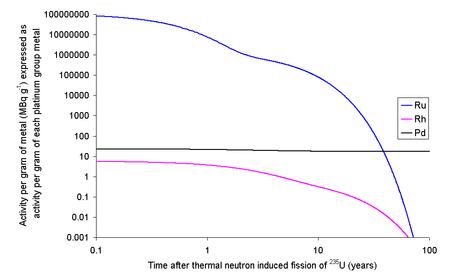
Ruthenium
Each kilogram of the fission products of 235U will contain 63.44 grams of ruthenium isotopes with halflives longer than a day. Since a typical used nuclear fuel contains about 3% fission products, one ton of used fuel will contain about 1.9 kg of ruthenium. The 103Ru and 106Ru will render the fission ruthenium very radioactive. If the fission occurs in an instant then the ruthenium thus formed will have an activity due to 103Ru of 109 TBq g−1 and 106Ru of 1.52 TBq g−1. 103Ru has a half-life of about 39 days meaning that within 390 days it will have effectively decayed to the only stable isotope of rhodium, 103Rh, well before any reprocessing is likely to occur. 106Ru has a half-life of about 373 days, meaning that if the fuel is left to cool for 5 years before reprocessing only about 3% of the original quantity will remain; the rest will have decayed.[1] To put the values in the table into perspective, the activity in natural potassium (due to naturally occurring 40
K) is about 30 Bq per gram.
Rhodium
It is possible to extract rhodium from used nuclear fuel: 1 kg of fission products of 235U contains 13.3 grams of 103Rh. At 3% fission products by weight, one ton of used fuel will contain about 400 grams of rhodium. The longest lived radioisotope of rhodium is 102mRh with a half-life of 2.9 years, while the ground state (102Rh) has a half-life of 207 days.[1]
Each kilogram of fission rhodium will contain 6.62 ng of 102Rh and 3.68 ng of 102mRh. As 102Rh decays by beta decay to either 102Ru (80%) (some positron emission will occur) or 102Pd (20%) (some gamma ray photons with about 500 keV are generated) and the excited state decays by beta decay (electron capture) to 102Ru (some gamma ray photons with about 1 MeV are generated). If the fission occurs in an instant then 13.3 grams of rhodium will contain 67.1 MBq (1.81 mCi) of 102Rh and 10.8 MBq (291 μCi) of 102mRh. As it is normal to allow used nuclear fuel to stand for about five years before reprocessing, much of this activity will decay away leaving 4.7 MBq of 102Rh and 5.0 MBq of 102mRh. If the rhodium metal was then left for 20 years after fission, the 13.3 grams of rhodium metal would contain 1.3 kBq of 102Rh and 500 kBq of 102mRh. Rhodium has the highest price of these precious metals ($25,000/kg in 2015), but the cost of the separation of the rhodium from the other metals needs to be considered.[1]
Palladium
Palladium is also produced by nuclear fission in small percentages, amounting to 1 kg per ton of spent fuel. As opposed to rhodium and ruthenium, palladium has a radioactive isotope, 107Pd, with a very long half-life (6.5 million years), so palladium produced in this way has a very low radioactive intensity. Mixed in with the other isotopes of palladium recovered from the spent fuel, this gives a radioactive dose rate of 7.207×10−5 Ci, which is well below the safe level of 1×10−3 Ci.[clarification needed] Also, 107Pd has a very low decay energy of only 33 keV, and so would be unlikely to pose a hazard even if pure. But, the other isotope of Pd, 108Pd , is not radioactive and popular( 26,46%, under 106Pd with 27,33%).
Silver
Silver is produced as result of nuclear fission in small amounts (approximately 0.1%). The vast majority of produced Silver is Ag-109 which is stable, and Ag 111 which decays away very quickly to form Cd 111. The only radioactive isotopes with a significant half lives produced are the metastable Ag-110m (249.8 d) and Ag-108m (418 years), the former of which is produced via neutron capture from Ag-109), and the latter of which is only formed in trace quantities. After a short period in storage the produced Silver is almost entirely stable and safe to use. Because of the modest price of silver, extraction of silver alone from highly radioactive fission products would be uneconomical. When recovered with ruthenium, rhodium, and palladium (price of silver in 2011: about 880 €/kg; rhodium; and ruthenium: about 30,000 €/kg) the economics change substantially: silver becomes a byproduct of platinoid metal recovery from fission waste and the marginal cost of processing the byproduct could be competitive.
Precious metals produced via irradiation
Ruthenium
In addition to being a fission product of uranium, as described above, another way to produce ruthenium is to start with molybdenum, which has a price averaging between $10 and $20/kg, in contrast with ruthenium's $1860/kg.[2] The isotope 100Mo, which has an abundance of 9.6% in natural molybdenum, can be transmuted to 101Mo by slow neutron irradiation. 101Mo and its daughter product, 101Tc, both have beta-decay half-lives of roughly 14 minutes. The end product is stable 101Ru. Alternately, it can be produced by the neutron inactivation of 99Tc; the resulting 100Tc has a half-life of 16 seconds and decays to the stable 100Ru. Given that Technetium-99 is among the most problematic long-lived fission products and - unlike its nuclear isomer 99m
Tc - has no known applications, production of Ruthenium from nuclear waste derived Technetium appears particularly promising. However, if Ruthenium that can be used without having to wait for nuclear decays to occur is desired, a particularly isotopically and chemically pure Technetium-99 target is needed.99m
Tc has important medical applications and the production of 99
Tc waste from it is unavoidable. If Ruthenium is produced from such a source, a relatively pure 99
Tc feedstock can be guaranteed and it might be possible to generate economic benefit from both the waste disposal of 99
Tc and the subsequent sale of Ruthenium.
Rhodium
In addition to being a fission product of uranium, as described above, another way to produce rhodium is to start with ruthenium, which has a price of $1860/kg, which is much lower than rhodium's $765,188/kg. The isotope 102Ru, which forms 31.6% of natural ruthenium, can be transmuted to 103Ru by slow neutron irradiation. 103Ru then decays to 103Rh via beta decay, with a half-life of 39.26 days. The isotopes 98Ru through 101Ru, which together form 44.2% of natural ruthenium, could also be transmuted into 102Ru, and subsequently to 103Ru and then 103Rh, through multiple neutron captures in a nuclear reactor. As Ruthenium can also be produced from lower value feedstocks such as Technetium or Molybdenum (as described above) it might be possible to produce very high value Rhodium via successive neutron capture (and beta decays) from low value molybdenum or even "waste" Technetium.
Rhenium
The cost of rhenium as of January 2010 was $6,250/kg; by contrast, tungsten is very cheap, with a price of under $30/kg as of July 2010.[3] The isotopes 184W and 186W together make up roughly 59% of naturally-occurring tungsten. Slow-neutron irradiation could convert these isotopes into 185W and 187W, which have half-lives of 75 days and 24 hours, respectively, and always undergo beta decay to the corresponding rhenium isotopes.[4][5] These isotopes could then be further irradiated to transmute them into osmium (see below), increasing their value further. Also, 182W and 183W, which together form 40.8% of naturally-occurring tungsten, can, via multiple neutron captures in a nuclear reactor, be transmuted into 184W, which can then be transmuted into rhenium.
Osmium
The cost of osmium as of January 2010 was $12,217 per kilogram, making it roughly twice the price of rhenium, which is worth $6,250/kg. Rhenium has two naturally occurring isotopes, 185Re and 187Re. Irradiation by slow neutrons would transmute these isotopes into 186Re and 188Re, which have half-lives of 3 days and 17 hours, respectively. The predominant decay pathway for both of these isotopes is beta-minus decay into 186Os and 188Os.[6][7]
Iridium
The cost of iridium as of January 2010 was $13,117/kg, somewhat higher than that of osmium ($12,217/kg). The isotopes 190Os and 192Os together make up roughly 67% of naturally-occurring osmium. Slow-neutron irradiation could convert these isotopes into 191Os and 193Os, which have half-lives of 15.4 and 30.11 days, respectively, and always undergo beta decay to 191Ir and 193Ir, respectively.[8][9] Also, 186Os through 189Os could be transmuted into 190Os through multiple neutron captures in a nuclear reactor, and subsequently into iridium. These isotopes could then be further irradiated to transmute them into platinum (see below), increasing their value further.
Platinum
The cost of platinum as of October 2014 was $39,900 per kilogram, making it equally as expensive as rhodium. Iridium, by contrast, has only about half the value of platinum ($18,000/kg). Iridium has two naturally occurring isotopes, 191Ir and 193Ir. Irradiation by slow neutrons would transmute these isotopes into 192Ir and 194Ir, with short half-lives of 73 days and 19 hours, respectively; the predominant decay pathway for both of these isotopes is beta-minus decay into 192Pt and 194Pt.[10][11]
Cobalt
Cobalt, despite having 110 tons-production per year, is also manufactured from iron. Firstly, isotopes of iron by slow neutrons would transmute these isotopes into 59Fe, which has a half-life is 44.6 days. Its decay pathway for this isotope is beta-minus decay into 59Co. Although this method is not too efficient, it provides the method to ensure that the wars and conflicts in Africa would stop in the near future.
Gold
Chrysopoeia, the artificial production of gold, is the symbolic goal of alchemy. Such transmutation is possible in particle accelerators or nuclear reactors, although the production cost is currently many times the market price of gold. Since there is only one stable gold isotope, 197Au, nuclear reactions must create this isotope in order to produce usable gold.
Gold synthesis in an accelerator
Gold synthesis in a particle accelerator is possible in many ways. The Spallation Neutron Source has a liquid mercury target which will be transmuted into gold, platinum, and iridium, which are lower in atomic number than mercury.[citation needed]
Gold synthesis in a nuclear reactor
Gold was synthesized from mercury by neutron bombardment in 1941, but the isotopes of gold produced were all radioactive.[12] In 1924, a Japanese physicist, Hantaro Nagaoka, accomplished the same feat.[13]
Gold can currently be manufactured in a nuclear reactor by the irradiation of either platinum or mercury.
Only the mercury isotope 196Hg, which occurs with a frequency of 0.15% in natural mercury, can be converted to gold by slow neutron capture, and following electron capture, decay into gold's only stable isotope, 197Au. When other mercury isotopes are irradiated with slow neutrons, they also undergo neutron capture, but either convert into each other or beta decay into the thallium isotopes 203Tl and 205Tl.
Using fast neutrons, the mercury isotope 198Hg, which composes 9.97% of natural mercury, can be converted by splitting off a neutron and becoming 197Hg, which then decays into stable gold. This reaction, however, possesses a smaller activation cross-section and is feasible only with unmoderated reactors.
It is also possible to eject several neutrons with very high energy into the other mercury isotopes in order to form 197Hg. However such high-energy neutrons can be produced only by particle accelerators.[clarification needed].
In 1980, Glenn Seaborg transmuted several thousand atoms of bismuth into gold at the Lawrence Berkeley Laboratory. His experimental technique was able to remove protons and neutrons from the bismuth atoms. Seaborg's technique was far too expensive to enable the routine manufacture of gold but his work is the closest yet to emulating the mythical Philosopher's Stone.[14][15]
Other methods
While in general nuclear decay cannot be sped up, reversed, slowed down or halted by non-nuclear means, some forms of beta decay including electron capture and bound state beta decay will not occur in fully ionized material ("stripped ions"). One of the more extreme cases is Dysprosium-163 which is stable in the neutral state but decays with a half life of 47 days to Holmium-163 when fully ionized. Neutral (non-ionized) Holmium-163 meanwhile decays by electron capture to Dysprosium-163. This is a rare example both of a nuclear reaction affected by the electron shell and of a reversible nuclear reaction.
Osmium production
Rhenium is one of only a few elements with stable isotopes whose most abundant isotope is unstable. Under normal conditions, the beta decay of 187
Re has such a high half life (4.12*10^10 years) as to be negligible. However, the fully ionized atom will undergo bound state beta decay much more quickly with a half life of "only" 32.9 years. The product is stable 187
Os in either case. Given the higher price of Osmium compared to Rhenium, this could be financially lucrative, especially since no particle accelerators or nuclear fission are involved in the process. Ionization energy rises with the positive charge of the atom - to remove Rhenium's first electron requires 7.8335 Electronvolt, to remove the second another 13 electronvolt and so on. In all, Rhenium has 75 electrons and all must be removed to observe the effect. Furthermore, plasma recombination must be avoided until the desired number of decays has occurred. However, the ionization requires still orders of magnitude less energy than producing a single neutron from nuclear spallation, which requires on the order of 30-50 Megaelectronvolt per Neutron.[16][17]
See also
References
- ^ a b c d Bush, R. P. (1991). "Recovery of Platinum Group Metals from High Level Radioactive Waste" (PDF). Platinum Metals Review. 35 (4): 202–208.
- ^ "Molybdenum Price". Retrieved July 25, 2010.
- ^ "Tungsten Prices".
- ^ "Tungsten-185".
- ^ "Tungsten-187".
- ^ "Rhenium-186".
- ^ "Rhenium-188".
- ^ "Osmium-191".
- ^ "Osmium-193".
- ^ "Iridium 194".
- ^ "Iridium 192".
- ^ R. Sherr; K. T. Bainbridge & H. H. Anderson (1941). "Transmutation of Mercury by Fast Neutrons". Physical Review. 60 (7): 473–479. Bibcode:1941PhRv...60..473S. doi:10.1103/PhysRev.60.473.
- ^ A.Miethe, ”Der Zerfall des Quecksilberatoms,” Naturwissenschaften, 12(1924): 597-598
- ^ Aleklett, K.; Morrissey, D.; Loveland, W.; McGaughey, P.; Seaborg, G. (1981). "Energy dependence of 209Bi fragmentation in relativistic nuclear collisions". Physical Review C. 23 (3): 1044. Bibcode:1981PhRvC..23.1044A. doi:10.1103/PhysRevC.23.1044.
- ^ Matthews, Robert (December 2, 2001). "The Philosopher's Stone". The Daily Telegraph. Retrieved September 22, 2020.
- ^ https://www3.aps.anl.gov/News/Meetings/Beams_and_Applications_Seminars/2005/VG20051028_Cousineau.pdf
- ^ https://accelconf.web.cern.ch/e96/PAPERS/ORALS/TUY04A.PDF
External links
- Spallation Neutron Source
- Mercury 197
- Mercury 197 decays to Gold 197
- Kolarik, Zdenek; Renard, Edouard V. (2005). "Potential Applications of Fission Platinoids in Industry" (PDF). Platinum Metals Review. 49 (2): 79. doi:10.1595/147106705X35263.
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part I PART I: General Considerations and Basic Chemistry" (PDF). Platinum Metals Review. 47 (2): 74–87.
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part II: Separation Process" (PDF). Platinum Metals Review. 47 (2): 123–131.
