Ionic compound: Difference between revisions
No edit summary |
|||
| Line 45: | Line 45: | ||
==Nomenclature== |
==Nomenclature== |
||
According to the [[IUPAC]], an ionic compound's common name is written using two words. The name of the cation comes first with the [[oxidation number]] written in parenthesis, followed by the name of the anion. For example, Fe<sub>2</sub>(SO<sub>4)<sub>3</sub> is named as Iron(III) Sulfate. |
According to the [[IUPAC]], an ionic compound's common name is written using two words. The name of the cation comes first with the [[oxidation number]] written in parenthesis, followed by the name of the anion. For example, Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> is named as Iron(III) Sulfate. |
||
[[Category:Chemical compounds]] |
[[Category:Chemical compounds]] |
||
Revision as of 06:27, 5 October 2007
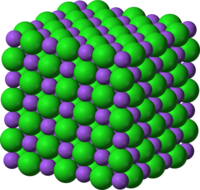
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. The positively charged ion is usually a metal ion and the negatively charged ion is non-metallic element or molecule.
Ions can be single atoms, as in common table salt sodium chloride, or more complex groups such as calcium carbonate. But to be considered an ion, they must carry a positive or negative charge. Thus, in an ionic bond, one 'bonder' must have a positive charge and the other a negative one. By sticking to each other, they resolve, or partially resolve, their separate charge imbalances. Positive to positive and negative to negative ionic bonds do not occur. (For a real world analogy, experiment with a pair of bar magnets.)
Chemical compounds are rarely strictly ionic or strictly covalent. Except for the most electronegative/electropositive pairs such as cesium fluoride, ionic compounds usually exhibit a degree of covalency. Similarly, covalent compounds often exhibit charge separations. See also HSAB theory.
Physical properties of ionic and molecular compounds:
| Ionic compounds | Molecular compounds | |
|---|---|---|
| States (at RTP) | Solid | Can be solid, liquid or gas at room temperature |
| Electrical conductivity | Solid: no Molten: yes |
No |
| Boiling point | High | Low |
| Solubility in water | Often high | Variable; usually lower than ionic |
| Thermal conductivity | Low | Low |
Characteristics
Ionic compounds have strong electrostatic bonds between particles. As a result, they generally have high melting and boiling points. They have good electrical conductivity when molten or in aqueous solution. While ionic inorganic compounds are solids at room temperature and will usually form crystals, organic ionic liquids are increasingly of interest.
Solubility
Following the aphorism, "like dissolves like", ionic compounds dissolve in polar solvents, especially those which ionize, such as water and ionic liquids. They are usually appreciably soluble in other polar solvents such as alcohols, acetone and dimethyl sulfoxide as well. Ionic compounds tend not to dissolve in nonpolar solvents such as diethyl ether or petrol.
When an ionic compound is named, the cation is named first and then the anion. When an elemental anion is named, the suffix, -ide, is added to the name of the element. There are two common types of cations: Type I and Type II. Type I cations have only one charge and their name is simply listed when the compound is named. Type II cations have more than one charge and when the ionic compound is named, a Roman numeral is used to denote the charge of the cation. In addition, there are common polyatomic anions which do not have suffixes in their name such as hypochlorite (ClO–).
Nomenclature
According to the IUPAC, an ionic compound's common name is written using two words. The name of the cation comes first with the oxidation number written in parenthesis, followed by the name of the anion. For example, Fe2(SO4)3 is named as Iron(III) Sulfate.
