Triphenylmethyl radical: Difference between revisions
→Miscellany: corrected date |
fixed minor error in descriptions |
||
| Line 5: | Line 5: | ||
Solutions containing the radical are [[yellow]] and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following [[Le Chatelier's principle]]. Conversely when the solution is cooled it becomes less yellow. |
Solutions containing the radical are [[yellow]] and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following [[Le Chatelier's principle]]. Conversely when the solution is cooled it becomes less yellow. |
||
When exposed to air the radical rapidly oxidizes to the [[peroxide]] (''Scheme 2'') and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with [[iodine]] to |
When exposed to air the radical rapidly oxidizes to the [[peroxide]] (''Scheme 2'') and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with [[iodine]] to hexaphenylmethyliodide. |
||
[[Image:Triphenylmethyl radical oxidation.png|300px|center|Scheme 2 |
[[Image:Triphenylmethyl radical oxidation.png|300px|center|Scheme 2 Hexaphenylmethyl radical oxidation]] |
||
The radical was discovered by [[Moses Gomberg]] in 1900.<ref>{{cite journal | title = An instance of trivalent carbon: triphenylmethyl | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1900 | volume = 22 | issue = 11 | pages = 757-771 | doi = 10.1021/ja02049a006}}</ref><ref>{{cite journal | title = On trivalent carbon | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1901 | volume = 23 | issue = 7 | pages = 496-502 | doi = 10.1021/ja02033a015}} (Note: radical is also called a ''cadicle'')</ref><ref>{{cite journal | title = On trivalent carbon | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1902 | volume = 24 | issue = 7 | pages = 597-628 | doi = 10.1021/ja02021a001}}</ref> He tried to prepare |
The radical was discovered by [[Moses Gomberg]] in 1900.<ref>{{cite journal | title = An instance of trivalent carbon: triphenylmethyl | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1900 | volume = 22 | issue = 11 | pages = 757-771 | doi = 10.1021/ja02049a006}}</ref><ref>{{cite journal | title = On trivalent carbon | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1901 | volume = 23 | issue = 7 | pages = 496-502 | doi = 10.1021/ja02033a015}} (Note: radical is also called a ''cadicle'')</ref><ref>{{cite journal | title = On trivalent carbon | author = [[Moses Gomberg|M. Gomberg]] | journal = [[J. Am. Chem. Soc.]] | year = 1902 | volume = 24 | issue = 7 | pages = 597-628 | doi = 10.1021/ja02021a001}}</ref> He tried to prepare triphenylethane from hexaphenylmethylchlorate and [[zinc]] in [[benzene]] in a [[Wurtz reaction]] and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated. |
||
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure '''4''' in scheme 1 <ref>{{cite journal | title = The hexaphenylethane riddle | author = J. M. McBride | journal = [[Tetrahedron (journal)|Tetrahedron]] | volume = 30 | issue = 14 | year = 1974 | pages = 2009-2022 | doi = 10.1016/S0040-4020(01)97332-6}}</ref>. It subsequently took until 1968 for its rediscovery when researchers at the [[Vrije Universiteit Amsterdam]] published [[proton NMR]] data <ref>{{cite journal | title = A new interpretation of the monomer-dimer equilibrium of triphenylmethyl- and alkylsubstituted-diphenyl methyl-radicals in solution | author = H. Lankamp, W. Th. Nauta and C. MacLean | journal = [[Tetrahedron Letters]] | volume = 9 | issue = 2 |year = 1968 | pages = 249-254 | doi = 10.1016/S0040-4039(00)75598-5}}</ref>. In hindsight the substituted ethane molecule does not make sense at all because it is simply too [[steric hindrance|sterically]] overcrowded. |
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure '''4''' in scheme 1 <ref>{{cite journal | title = The hexaphenylethane riddle | author = J. M. McBride | journal = [[Tetrahedron (journal)|Tetrahedron]] | volume = 30 | issue = 14 | year = 1974 | pages = 2009-2022 | doi = 10.1016/S0040-4020(01)97332-6}}</ref>. It subsequently took until 1968 for its rediscovery when researchers at the [[Vrije Universiteit Amsterdam]] published [[proton NMR]] data <ref>{{cite journal | title = A new interpretation of the monomer-dimer equilibrium of triphenylmethyl- and alkylsubstituted-diphenyl methyl-radicals in solution | author = H. Lankamp, W. Th. Nauta and C. MacLean | journal = [[Tetrahedron Letters]] | volume = 9 | issue = 2 |year = 1968 | pages = 249-254 | doi = 10.1016/S0040-4039(00)75598-5}}</ref>. In hindsight the substituted ethane molecule does not make sense at all because it is simply too [[steric hindrance|sterically]] overcrowded. |
||
Revision as of 04:42, 19 March 2008
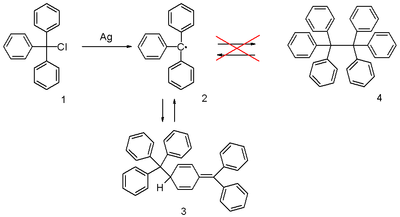
The triphenylmethyl radical is a persistent radical and the first ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethylchloride 1 (scheme 1) by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type dimer 3. In benzene the concentration of the radical is 2% [1].
Solutions containing the radical are yellow and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following Le Chatelier's principle. Conversely when the solution is cooled it becomes less yellow.
When exposed to air the radical rapidly oxidizes to the peroxide (Scheme 2) and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to hexaphenylmethyliodide.
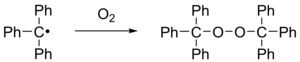
The radical was discovered by Moses Gomberg in 1900.[2][3][4] He tried to prepare triphenylethane from hexaphenylmethylchlorate and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure 4 in scheme 1 [5]. It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR data [6]. In hindsight the substituted ethane molecule does not make sense at all because it is simply too sterically overcrowded.
Miscellany
- Gomberg concluded his 1900 article with the sentence "This work will be continued and I wish to reserve the field for myself." He ended his 1901 article by writing, "It is my intention to extend this study to other oxygen compounds, as well as to nitrogen derivatives, and I beg to reserve this field for further work." It is true that nineteenth-century chemists did not intrude on each other's research; to his dismay, Gomberg found out that this was not the case in the twentieth century.
External links
- Molecule of the Month June 1997 Link
- Experimental procedures Link
References
- ^ Advanced Organic Chemistry J. March, John Wiley & Sons ISBN 0-471-88841-9
- ^ M. Gomberg (1900). "An instance of trivalent carbon: triphenylmethyl". J. Am. Chem. Soc. 22 (11): 757–771. doi:10.1021/ja02049a006.
- ^ M. Gomberg (1901). "On trivalent carbon". J. Am. Chem. Soc. 23 (7): 496–502. doi:10.1021/ja02033a015. (Note: radical is also called a cadicle)
- ^ M. Gomberg (1902). "On trivalent carbon". J. Am. Chem. Soc. 24 (7): 597–628. doi:10.1021/ja02021a001.
- ^ J. M. McBride (1974). "The hexaphenylethane riddle". Tetrahedron. 30 (14): 2009–2022. doi:10.1016/S0040-4020(01)97332-6.
- ^ H. Lankamp, W. Th. Nauta and C. MacLean (1968). "A new interpretation of the monomer-dimer equilibrium of triphenylmethyl- and alkylsubstituted-diphenyl methyl-radicals in solution". Tetrahedron Letters. 9 (2): 249–254. doi:10.1016/S0040-4039(00)75598-5.
