Hydrogen fluoride: Difference between revisions
control |
mNo edit summary |
||
| Line 41: | Line 41: | ||
|} |
|} |
||
'''Hydrogen fluoride''' is a [[chemical compound]] with the [[chemical formula|formula]] HF. It is the principal industrial source of [[fluorine]], often in the aqueous form as [[hydrofluoric acid]], and thus is the precursor to many important compounds including pharmaceuticals and polymers (e.g. [[Teflon]]). HF is widely used in the petrochemical industry and a component of many [[superacid]]s. HF boils just below room temperature whereas the other hydrogen [[halide]]s condense at much lower temperatures. Unlike the other halogen hydrides, HF is lighter than air and its odour is particularly penetrating. Aqueous |
'''Hydrogen fluoride''' is a [[chemical compound]] with the [[chemical formula|formula]] HF. It is the principal industrial source of [[fluorine]], often in the aqueous form as [[hydrofluoric acid]], and thus is the precursor to many important compounds including pharmaceuticals and polymers (e.g. [[Teflon]]). HF is widely used in the petrochemical industry and a component of many [[superacid]]s. HF boils just below room temperature whereas the other hydrogen [[halide]]s condense at much lower temperatures. Unlike the other halogen hydrides, HF is lighter than air and its odour is particularly penetrating. Aqueous solution of HF, called [[hydrofluoric acid]], is strongly corrosive. |
||
==Structure== |
==Structure== |
||
Revision as of 02:04, 11 November 2008
| Hydrogen fluoride | |||||
|---|---|---|---|---|---|
| |||||
| Other names | Hydrogen fluoride Fluoric acid Hydrofluoride Hydrofluoric acid Fluorine monohydride | ||||
| Molecular formula | HF | ||||
| Molecular mass | 20.01 g/mol | ||||
| Physical state | Liquid/Gas | ||||
| CAS number | 7664-39-3 | ||||
| Density | 0.922 kg m-3 | ||||
| Solubility (water) | miscible | ||||
| Melting point | -84 °C (190 K, -118 °F) | ||||
| Boiling point | 19.54 °C (293 K, 67.2 °F) | ||||
| NFPA 704 |
| ||||
| Disclaimer and references | |||||
Hydrogen fluoride is a chemical compound with the formula HF. It is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers (e.g. Teflon). HF is widely used in the petrochemical industry and a component of many superacids. HF boils just below room temperature whereas the other hydrogen halides condense at much lower temperatures. Unlike the other halogen hydrides, HF is lighter than air and its odour is particularly penetrating. Aqueous solution of HF, called hydrofluoric acid, is strongly corrosive.
Structure
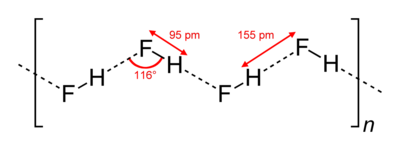
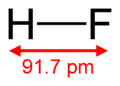
HF forms orthorhombic crystals, consisting of zig-zag chains of HF molecules. The HF molecules, with a short H-F bond of 0.95 Å, are linked to neighboring molecules by intermolecular H--F distances of 1.55 Å.[1]
Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.[2] The higher boiling point of HF relative to analogous species, such as HCl, is attributed to hydrogen bonding between HF molecules, as indicated by the existence of chains even in the liquid state.
Acidity
Dilute aqueous HF solutions are weakly acidic in contrast to corresponding solutions of the other hydrogen halides. A qualitative explanation for this behavior is related to the tendency of HF to hydrogen-bond and form ion-pair clusters such as F-·H3O+.[3][4]
In concentrated hydrogen fluoride solution, F− ions forms a [HF2]−(aq) complex with HF molecules. HF molecules remaining ionize to compensate the loss of F− ions. More H+ ions are thus formed, making concentrated HF an effectively strong acid.
Anhydrous hydrogen fluoride is an extremely strong acid (H0 ~ -11), comparable in strength to anhydrous sulfuric acid (H0 ~ -12).
Uses
HF is used for fluorinating polymers giving fluorocarbons, petroleum refining, glassmaking, aluminium manufacturing, titanium pickling, quartz purification, and metal finishing. It is also used to synthesize UF6, which is key to separating uranium isotopes.
Hydrogen fluoride can be found in consumer products for removing rust, cleaning brass, and glass etching, although use in consumer products is discouraged [citation needed] due to HF's corrosiveness and toxicity. Hydrogen fluoride is typically marketed in three common forms: anhydrous HF, aqueous 70% HF, aqueous 49% HF. HF is manufactured by the reaction of calcium fluoride (fluorspar) and sulfuric acid:
- CaF2 + H2SO4 → CaSO4 + 2 HF
The vapors from this reaction are a mixture of hydrogen fluoride, sulfuric acid, and a few minor byproducts.
Health effects
Upon contact with moisture, including tissue, hydrogen fluoride immediately converts to hydrofluoric acid, which is highly corrosive and toxic, and requires immediate medical attention.
References
- ^ Johnson, M. W.; Sándor, E.; Arzi, E. (1975). "The Crystal Structure of Deuterium Fluoride". Acta Crystallographica. B31: pages 1998–2003. doi:10.1107/S0567740875006711.
{{cite journal}}:|pages=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Mclain, Sylvia E. (2004). "On the Structure of Liquid Hydrogen Fluoride". Angewandte Chemie, International Edition. 43: 1952–55. doi:10.1002/anie.200353289.
- ^ Giguere, Paul A. (1980). "The nature of hydrofluoric acid. A spectroscopic study of the proton-transfer complex H3O+...F−". J. Am. Chem. Soc. 102: 5473. doi:10.1021/ja00537a008.
- ^ Radu Iftimie, Vibin Thomas, Sylvain Plessis, Patrick Marchand, and Patrick Ayotte (2008). "Spectral Signatures and Molecular Origin of Acid Dissociation Intermediates". J. Am. Chem. Soc. 130: 5901. doi:10.1021/ja077846o.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- "ATSDR - MMG: Hydrogen Fluoride". Retrieved May 14, 2006
- Barbalace, Kenneth. "Chemical Database - Hydrogen Fluoride. EnvironmentalChemistry.com". 1995 - 2006. Retrieved May 14, 2006
- Honeywell, Industrial Fluorines G525-521, "Recommended Medical Treatment for Hydrofluoric Acid Exposure"
- Cotton, F. A. and Wilkinson, G., Advanced Inorganic Chemistry, John Wiley and Sons: New York, 1988. ISBN 0471849979

