Immunohistochemistry: Difference between revisions
No edit summary |
changed FITC to fluorescein (base molecule), replaced Texas Red (also a rhodamine) with Dylight (similar to Alexa Fluor) |
||
| Line 1: | Line 1: | ||
[[image:immunohistochemistry.png|thumb|Immunohistochemistry labels individual proteins, such as [[Tyrosine_hydroxylase|TH]] (green) in the [[axons]] of sympathetic [[Autonomic_Nervous_System|autonomic]] neurons.]] |
[[image:immunohistochemistry.png|thumb|Immunohistochemistry labels individual proteins, such as [[Tyrosine_hydroxylase|TH]] (green) in the [[axons]] of sympathetic [[Autonomic_Nervous_System|autonomic]] neurons.]] |
||
'''Immunohistochemistry''' or '''IHC''' refers to the process of localizing proteins in cells of a tissue section exploiting the principle of [[antibody|antibodies]] binding specifically to [[antigen]]s in [[biological tissue]]s. <ref name="JA Ramos-Vara">{{cite journal | last = Ramos-Vara | first = JA | title = Technical Aspects of Immunohistochemistry | journal = Vet Pathol | volume = 42 | pages = 405–426 | year = 2005 | url = http://www.vetpathology.org/cgi/content/short/42/4/405 | doi = 10.1354/vp.42-4-405 | pmid = 16006601}}</ref> It takes its name from the roots "immuno," in reference to antibodies used in the procedure, and "histo," meaning tissue (compare to [[immunocytochemistry]]). Immunohistochemical staining is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death ([[apoptosis]]). IHC is also widely used in basic research to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue. |
'''Immunohistochemistry''' or '''IHC''' refers to the process of localizing proteins in cells of a tissue section exploiting the principle of [[antibody|antibodies]] binding specifically to [[antigen]]s in [[biological tissue]]s. <ref name="JA Ramos-Vara">{{cite journal | last = Ramos-Vara | first = JA | title = Technical Aspects of Immunohistochemistry | journal = Vet Pathol | volume = 42 | pages = 405–426 | year = 2005 | url = http://www.vetpathology.org/cgi/content/short/42/4/405 | doi = 10.1354/vp.42-4-405 | pmid = 16006601}}</ref> It takes its name from the roots "immuno," in reference to antibodies used in the procedure, and "histo," meaning tissue (compare to [[immunocytochemistry]]). Immunohistochemical staining is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death ([[apoptosis]]). IHC is also widely used in basic research to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue. |
||
Visualising an antibody-antigen interaction can be accomplished in a number of ways. In the most common instance, an antibody is conjugated to an enzyme, such as [[peroxidase]], that can catalyse a colour-producing reaction ''(see [[immunoperoxidase|immunoperoxidase staining]])''. Alternatively, the antibody can also be tagged to a [[fluorophore]], such as [[ |
Visualising an antibody-antigen interaction can be accomplished in a number of ways. In the most common instance, an antibody is conjugated to an enzyme, such as [[peroxidase]], that can catalyse a colour-producing reaction ''(see [[immunoperoxidase|immunoperoxidase staining]])''. Alternatively, the antibody can also be tagged to a [[fluorophore]], such as [[fluorescein]], [[rhodamine]], [[DyLight Fluor]] or [[Alexa Fluor]] ''(see [[immunofluorescence]])''. |
||
==Antibody types== |
==Antibody types== |
||
Revision as of 18:57, 30 April 2009
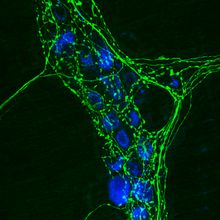
Immunohistochemistry or IHC refers to the process of localizing proteins in cells of a tissue section exploiting the principle of antibodies binding specifically to antigens in biological tissues. [1] It takes its name from the roots "immuno," in reference to antibodies used in the procedure, and "histo," meaning tissue (compare to immunocytochemistry). Immunohistochemical staining is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death (apoptosis). IHC is also widely used in basic research to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue. Visualising an antibody-antigen interaction can be accomplished in a number of ways. In the most common instance, an antibody is conjugated to an enzyme, such as peroxidase, that can catalyse a colour-producing reaction (see immunoperoxidase staining). Alternatively, the antibody can also be tagged to a fluorophore, such as fluorescein, rhodamine, DyLight Fluor or Alexa Fluor (see immunofluorescence).
Antibody types
The antibodies used for specific detection can be polyclonal or monoclonal. Monoclonal antibodies are generally considered to exhibit greater specificity. Polyclonal antibodies are made by injecting animals with peptide antigens, and then after a secondary immune response is stimulated, isolating antibodies from whole serum. Thus, polyclonal antibodies are a heterogeneous mix of antibodies that recognize several epitopes. Antibodies can also be classified as primary or secondary reagents. Primary antibodies are raised against an antigen of interest and are typically unconjugated (unlabelled), while secondary antibodies are raised against primary antibodies. Hence, secondary antibodies recognize immunoglobulins of a particular species and are conjugated to either biotin or a reporter enzyme such as alkaline phosphatase or horseradish peroxidase (HRP). Some secondary antibodies are conjugated to fluorescent agents, such as the Alexa Fluor or Dylight Fluor family, are also frequently used for detection of proteins in IHC procedures. Protein concentration is generally measured by densitometry analysis, where the intensity of staining correlates with the amount of the protein of interest.
Sample preparation
In the procedure, depending on the purpose and the thickness of the experimental sample, either thin (about 4-40 μm) slices are taken of the tissue of interest, or if the tissue is not very thick and is penetrable it is used whole. The slicing is usually accomplished through the use of a microtome, and slices are mounted on slides. "Free-floating IHC" uses slices that are not mounted, these slices are normally produced using a vibrating microtome.
Direct and indirect IHC
There are two strategies used for the immunohistochemical detection of antigens in tissue, the direct method and the indirect method. In both cases, many antigens also need an additional step for unmasking, which often makes the difference between staining and no staining. Unlike immunocytochemistry, the tissue does not need to be permeabilized because this has already been accomplished by the microtome blade during sample preparation. Detergents like Triton X-100 are generally used in immunohistochemistry to reduce surface tension, allowing less reagent to be used to achieve better and more even coverage of the sample.

Direct
The direct method is a one-step staining method, and involves a labeled antibody (e.g. FITC conjugated antiserum) reacting directly with the antigen in tissue sections. This technique utilizes only one antibody and the procedure is therefore simple and rapid. However, it can suffer problems with sensitivity due to little signal amplification and is in less common use than indirect methods.
Indirect=

The indirect method involves an unlabeled primary antibody (first layer) which reacts with tissue antigen, and a labeled secondary antibody (second layer) which reacts with the primary antibody. (The secondary antibody must be raised against the IgG of the animal species in which the primary antibody has been raised.) This method is more sensitive due to signal amplification through several secondary antibody reactions with different antigenic sites on the primary antibody. The second layer antibody can be labeled with a fluorescent dye or an enzyme. In a common procedure, a biotinylated secondary antibody is coupled with streptavidin-horseradish peroxidase. This is reacted with 3,3'-Diaminobenzidine (DAB) to produce a brown staining wherever primary and secondary antibodies are attached in a process known as DAB staining. The reaction can be enhanced using nickel, producing a deep purple/gray staining.
The indirect method, aside from its greater sensitivity, also has the advantage that only a relatively small number of standard conjugated (labeled) secondary antibodies needs to be generated. For example, a labeled secondary antibody raised against rabbit IgG, which can be purchased "off the shelf," is useful with any primary antibody raised in rabbit. With the direct method, it would be necessary to make custom labeled antibodies against every antigen of interest.
Diagnostic IHC markers

IHC is an excellent detection technique and has the tremendous advantage of being able to show exactly where a given protein is located within the tissue examined. It is also an effective way to examine the tissues .This has made it a widely-used technique in the neurosciences, enabling researchers to examine protein expression within specific brain structures. Its major disadvantage is that, unlike immunoblotting techniques where staining is checked against a molecular weight ladder, it is impossible to show in IHC that the staining corresponds with the protein of interest. For this reason, primary antibodies must be well-validated in a Western Blot or similar procedure. The technique is even more widely used in diagnostic surgical pathology for typing tumors (e.g. immunostaining for e-cadherin to differentiate between DCIS (ductal carcinoma in situ: stains positive) and LCIS (lobular carcinoma in situ: does not stain positive)[2]).
- Carcinoembryonic antigen (CEA): used for identification of adenocarcinomas. Not specific for site.
- Cytokeratins: used for identification of carcinomas but may also be expressed in some sarcomas.[3]
- CD15 and CD30 : used for Hodgkin's disease
- Alpha fetoprotein: for yolk sac tumors and hepatocellular carcinoma
- CD117 (KIT): for gastrointestinal stromal tumors (GIST)
- CD10 (CALLA): for renal cell carcinoma and acute lymphoblastic leukemia
- Prostate specific antigen (PSA): for prostate cancer
- estrogens and progesterone staining for tumour identification
- Identification of B-cell lymphomas using CD20
- Identification of T-cell lymphomas using CD3
Directing therapy
A variety of molecular pathways are altered in cancer and some of the alterations can be targeted in cancer therapy. Immunohistochemistry can be used to assess which tumors are likely to respond to therapy, by detecting the presence or elevated levels of the molecular target.
Chemical inhibitors
Tumor biology allows for a number of potential intracellular targets. Many tumors are hormone dependent. The presence of hormone receptors can be used to determine if a tumor is potentially responsive to antihormonal therapy. One of the first therapies was the antiestrogen, tamoxifen, used to treat breast cancer. Such hormone receptors can be detected by immunohistochemistry.[4] Imatinib, an intracellualar tyrosine kinase inhibitor, was developed to treat chronic myelogenous leukemia, a disease characterized by the formation of a specific abnormal tyrosine kinase. Imitanib has proven effective in tumors, that express other tyrosine kinases, most notably KIT. Most gastrointestinal stromal tumors express KIT, which can be detected by immunohistochemistry.[5]
Monoclonal antibodies
Many proteins shown to be highly upregulated in pathological states by immunohistochemistry are potential targets for therapies utilising monoclonal antibodies. Monoclonal antibodies, due to their size, are utilized against cell surface targets. Among the overexpressed targets, the members of the epidermal growth factor receptor (EGFR) family, transmembrane proteins with an extracellular receptor domain regulating an intracellular tyrosine kinase, [6] Of these, HER2/neu (also known as Erb-B2) was the first to be developed. The molecule is highly expressed in a variety of cancer cell types, most notably breast cancer. As such, antibodies against HER2/neu have been FDA approved for clinical treatment of cancer under the drug name Herceptin. There are commercially available immunohistochemical tests, Dako HercepTestand Ventana Pathway.[7] Similarly, EGFR (HER-1) is overexpressed is a variety of cancers including head and neck and colon. Immunohistochemistry is used to determined patients who may benefit from therapeutic antibodies such as Erbitux (cetuximab).[8] Commercial systems to detect EGFR by immunohistochemistry include the Dako pharmDx.
References
- ^ Ramos-Vara, JA (2005). "Technical Aspects of Immunohistochemistry". Vet Pathol. 42: 405–426. doi:10.1354/vp.42-4-405. PMID 16006601.
- ^ O'Malley F and Pinder S, Breast Pathology, 1st. Ed. Elsevier 2006. ISBN: 978-0-443-06680-1
- ^ Leader M, Patel J, Makin C, Henry K (1986). "An analysis of the sensitivity and specificity of the cytokeratin marker CAM 5.2 for epithelial tumours. Results of a study of 203 sarcomas, 50 carcinomas and 28 malignant melanomas". Histopathology. 10 (12): 1315–24. doi:10.1111/j.1365-2559.1986.tb02574.x. PMID 2434403.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jørgensen, Jan Trøst (2007). "Pharmacodiagnostics and targeted therapies - a rational approach for individualizing medical anticancer therapy in breast cancer". The Oncologist. 12 (4). United States: AlphaMed Press: 397–405. doi:10.1634/theoncologist.12-4-397. ISSN 1083-7159. PMID 17470682. Retrieved 2008-03-14.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Gold JS, Dematteo RP (2006). "Combined surgical and molecular therapy: the gastrointestinal stromal tumor model". Ann. Surg. 244 (2): 176–84. doi:10.1097/01.sla.0000218080.94145.cf. PMC 1602162. PMID 16858179.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Harari, P M (2004). "Epidermal growth factor receptor inhibition strategies in oncology". Endocrine-Related Cancer. 11 (4). England: Society for Endocrinology: 689–708. doi:10.1677/erc.1.00600. ISSN 1351-0088. PMID 15613446. Retrieved 2008-03-14.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Press, Michael F. (September 15, 2005). "Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials". Clinical Cancer Research. 2005 15, 11(18): (18). United States: American Association for Cancer Research.: 6598–6607. doi:10.1158/1078-0432.CCR-05-0636. ISSN 1078-0432. PMID 16166438. Retrieved 2008-03-14.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ Bibeau F, Boissière-Michot F, Sabourin JC; et al. (2006). "Assessment of epidermal growth factor receptor (EGFR) expression in primary colorectal carcinomas and their related metastases on tissue sections and tissue microarray". Virchows Arch. 449 (3): 281–7. doi:10.1007/s00428-006-0247-9. PMC 1888717. PMID 16865406.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Yale Core Tissue Microarray Facility
- Histochemical Staining Methods - University of Rochester Department of Pathology
- Immunohistochemistry Staining Protocols and Image Gallery
- Immunohistochemistry Staining Protocol
- HistoWiki entry for Immunohistochemistry
- Burnett R, Guichard Y, Barale E (1997). "Immunohistochemistry for light microscopy in safety evaluation of therapeutic agents: an overview". Toxicology. 119 (1): 83–93. doi:10.1016/S0300-483X(96)03600-1. PMID 9129199.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Immunohistochemistry at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Joyner, A. (2008). "Immunohistochemistry of Whole-Mount Mouse Embryos". Cold Spring Harbor Protocols. 2008: pdb.prot4820. doi:10.1101/pdb.prot4820.
