2,4,6-Trinitroaniline: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Construction}} |
|||
{{Chembox |
{{Chembox |
||
|ImageFile=Trinitroaniline structure.svg |
|ImageFile=Trinitroaniline structure.svg |
||
Revision as of 18:13, 17 March 2010
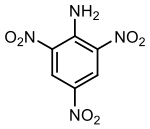
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-trinitroaniline
| |
| Other names
Picramide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.007.004 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H4N4O6 | |
| Molar mass | 228.12 g/mol |
| Appearance | yellow/orange/red powder |
| Density | 1.8 g/cm3 |
| Melting point | 370.0°F |
| Boiling point | explodes before boiling |
| insoluble | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
instantaneous explosion |
| Flash point | unknown |
| Explosive data | |
| Shock sensitivity | unknown |
| Friction sensitivity | unknown |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.[1] The appearance of Trinitroaniline varies from yellow to orange to red depending on its purity and concentration.
Applications/Uses
Trinitroaniline is only used in modern times in some small warheads in explosive devices such as mortars. In World War II it was famously used in the Yokosuka MXY-7 Ohka, a kamikaze antishipping vessel.
Health Concerns/Precautions
The primary hazard of working with Trinitroaniline is the risk of an instantaneous explosion, not flying projectiles or fragments. Trinitroaniline is dangerously explosive. Symptoms of exposure to this compound may include skin and eye irritation, headache, drowsiness, weakness, cyanosis, and respiratory distress. Chronic exposure may lead to weight loss, anemia, and possible liver damage. In case of minor fires with where only extremely small amounts of Trinitroaniline are present foam or CO2 extinguishers may be used. If any moderate or large amount is present, standard procedure is to evacuate the area and allow to burn.[2] Standard safety procedure is to keep sparks, flames, and other sources of ignition away and to keep the compound wet when at all times when its being worked with. If any is spilled, immediately wet down before attempting to clean up. Standard health precautions include not breathing dusts, and fumes from material especially if burning. Wear appropriate chemical protective gloves and goggles. Do not handle broken packages unless wearing appropriate personal protective equipment. Wash away any material which may have contacted the body with copious amounts of water or soap and water. Wear positive pressure self-contained breathing apparatus if fighting fires involving this material.[3]
References
