Tetryl: Difference between revisions
No edit summary |
No edit summary |
||
| Line 24: | Line 24: | ||
| ShockSens = Sensitive |
| ShockSens = Sensitive |
||
| FrictionSens = Sensitive |
| FrictionSens = Sensitive |
||
| ExplosiveV = 7,570 [[metre per second|m/s]] |
| ExplosiveV = 7,570 [[metre per second|m/s]], or 24,836 [[feet per second|f/s]] |
||
| REFactor = 1.25 |
| REFactor = 1.25 |
||
}} |
}} |
||
Revision as of 18:30, 19 March 2010
| |
| Names | |
|---|---|
| IUPAC name
N-methyl-N,2,4,6-tetranitroaniline
| |
| Other names
nitramine, tetralite, tetril
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.006.848 |
PubChem CID
|
|
| UN number | 0208 |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H5N5O8 | |
| Molar mass | 287.15 g/mol |
| Appearance | Odorless yellow crystalline solid |
| Density | 1.73 g/cm3 |
| Melting point | 129.5 °C (265.1 °F; 402.6 K) |
| Boiling point | Decomposes at 187 °C |
| virtually insoluble in H2O | |
| Explosive data | |
| Shock sensitivity | Sensitive |
| Friction sensitivity | Sensitive |
| RE factor | 1.25 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
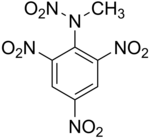
2,4,6-trinitrophenylmethylnitramine commonly refered to as Tetryl (C7H5N5O8) is a sensitive explosive compound used to make detonators and explosive booster charges.
Tetryl is a nitramine booster explosive, though the use has been largely superseded by RDX. Tetryl is sensitive secondary high explosive used as a booster, a small charge placed next to the detonator in order to propagate the detonation into the main charge. While it is commonly known as Tetryl it is in fact Trinitrophenylmethylnitramine (derivative of Benzene). This is a standard booster explosive. Tetryl is a fine yellow crystalline material. When tetryl is heated, it first melts, then decomposes and explodes. It burns readily and is more easily detonated than explosive D. [1]
It is a yellow crystalline solid powder material, practically insoluble in water but soluble in Acetone, Benzene and other solvents. It burns readily and is more easily detonated than TNT or Ammonium Picrate (Explosive D), being about as sensitive as Picric Acid. It is detonated by friction, shock, or spark . It remains stable at all temperatures which may be encountered in storage. It is generally used in the form of pressed pellets, and has been approved as the standard bursting charge for small-caliber projectiles, since it gives much better fragmentation than TNT. It also has greater shattering ability than any other military high explosive, and must be properly protected from bullet fire . Its rate of detonation is 23,600-23,900 feet per second. Tetryl is the basis for the service Tetryl blasting caps necessary for positive detonation of TNT. A mixture of Fulminate of Mercury and Potassium Chlorate is included in the cap to insure detonation of Tetryl.[2]
The most toxic ordnance compounds, tetryl and 1,3,5-TNB, are also the most degradable. Therefore these chemicals are expected to be short-lived in nature, and environmental impacts would not be expected in areas that are not currently subject to chronic inputs of these chemicals. Tetryl decomposes rapidly in methanol/water solutions, as well as with heat. All aqueous samples expected to contain tetryl should be diluted with acetonitrile prior to filtration and acidified to pH <3. All samples expected to contain tetryl should not be exposed to temperatures above room temperature. In addition, degradation products of tetryl appear as a shoulder on the 2,4,6-TNT peak. Peak heights rather than peak areas should be used when tetryl is present in concentrations that are significant relative to the concentration of 2,4,6-TNT.[3]
Tetryl was used mainly during World Wars I and II and later conflicts. Tetryl is usually used on its own, though can sometimes be found in compositions such as tetrytol. Tetryl is no longer manufactured or used in the United States, but can still be found in legacy munitions such as the M14 anti-personnel landmine.
Production
Tetryl is produced by simply slowly mixing dimethylaniline with concentrated nitric acid in the presence of sulfuric acid (the catalyst).
Health Concerns/Precautions
Although tetryl is among the most toxic explosive compounds, it is very short lived. This combined with the fact that the health impacts of this compound are largely unstudied, not much is known about any health problems that this compound may cause.
See also
References
- Cooper, Paul W., Explosives Engineering, New York: Wiley-VCH, 1996. ISBN 0-471-18636-8
