Debye model: Difference between revisions
→Derivation: Changed the word "side" (implying 2D) to "edge" in derivation of atomic separation. |
ArielGenesis (talk | contribs) m →Derivation: U is average enery, E is the total |
||
| Line 26: | Line 26: | ||
Let's now compute the total energy in the box, |
Let's now compute the total energy in the box, |
||
:<math> |
:<math>E = \sum_n E_n\,\bar{N}(E_n)\,,</math> |
||
where <math>\bar{N}(E_n)</math> is the number of phonons in the box with energy <math>E_n</math>. In other words, the total energy is equal to the sum of energy multiplied by the number of phonons with that energy (in one dimension). In 3 dimensions we have: |
where <math>\bar{N}(E_n)</math> is the number of phonons in the box with energy <math>E_n</math>. In other words, the total energy is equal to the sum of energy multiplied by the number of phonons with that energy (in one dimension). In 3 dimensions we have: |
||
Revision as of 20:37, 17 November 2010
| Statistical mechanics |
|---|
 |
In thermodynamics and solid state physics, the Debye model is a method developed by Peter Debye in 1912[1] for estimating the phonon contribution to the specific heat (heat capacity) in a solid. It treats the vibrations of the atomic lattice (heat) as phonons in a box, in contrast to the Einstein model, which treats the solid as many individual, non-interacting quantum harmonic oscillators. The Debye model correctly predicts the low temperature dependence of the heat capacity, which is proportional to – the Debye T3 law. Just like the Einstein model, it also recovers the Dulong-Petit law at high temperatures. But due to simplifying assumptions, its accuracy suffers at intermediate temperatures.
Derivation
The Debye model is a solid-state equivalent of Planck's law of black body radiation, where one treats electromagnetic radiation as a gas of photons in a box. The Debye model treats atomic vibrations as phonons in a box (the box being the solid). Most of the calculation steps are identical.
Consider a cube of side . From the particle in a box article, the resonating modes of the sonic disturbances inside the box (considering for now only those aligned with one axis) have wavelengths given by
where is an integer. The energy of a phonon is
where is Planck's constant and is the frequency of the phonon. Making the approximation that the frequency is inversely proportional to the wavelength, we have:
in which is the speed of sound inside the solid. In three dimensions we will use:
The approximation that the frequency is inversely proportional to the wavelength (giving a constant speed of sound) is good for low-energy phonons but not for high-energy phonons (see the article on phonons.) This is one of the limitations of the Debye model, and corresponds to uncorrectness of the results at intermediate temperatures, whereas both at low temperatures and also at high temperatures they are exact.
Let's now compute the total energy in the box,
where is the number of phonons in the box with energy . In other words, the total energy is equal to the sum of energy multiplied by the number of phonons with that energy (in one dimension). In 3 dimensions we have:
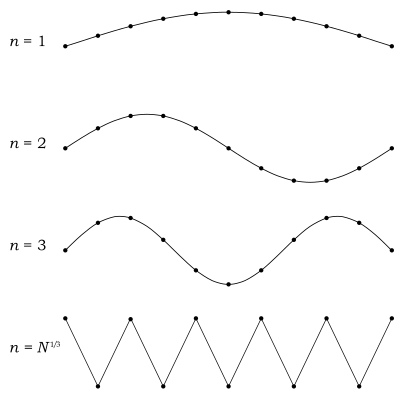
Now, this is where Debye model and Planck's law of black body radiation differ. Unlike electromagnetic radiation in a box, there is a finite number of phonon energy states because a phonon cannot have infinite frequency. Its frequency is bound by the medium of its propagation—the atomic lattice of the solid. Consider an illustration of a transverse phonon below.
It is reasonable to assume that the minimum wavelength of a phonon is twice the atom separation, as shown in the lower figure. There are atoms in a solid. Our solid is a cube, which means there are atoms per edge. Atom separation is then given by , and the minimum wavelength is
making the maximum mode number (infinite for photons)
This is the upper limit of the triple energy sum
For slowly-varying, well-behaved functions, a sum can be replaced with an integral (also known as Thomas-Fermi approximation)
So far, there has been no mention of , the number of phonons with energy Phonons obey Bose-Einstein statistics. Their distribution is given by the famous Bose-Einstein formula
Because a phonon has three possible polarization states (one longitudinal, and two transverse which approximately do not affect its energy) the formula above must be multiplied by 3,
(Actually one uses an effective sonic velocity , i.e. the Debye temperature (see below) is proportional to , more precisely , where one distinguishes longitudinal and transversal sound-wave velocities (contributions 1/3 and 2/3, respectively). The Debye temperature or the effective sonic velocity is a measure of the hardness of the crystal.)
Substituting this into the energy integral yields
The ease with which these integrals are evaluated for photons is due to the fact that light's frequency, at least semi-classically, is unbound. As the figure above illustrates, this is not true for phonons. In order to approximate this triple integral, Debye used spherical coordinates
and boldly approximated the cube by an eighth of a sphere
where is the radius of this sphere, which is found by conserving the number of particles in the cube and in the eighth of a sphere. The volume of the cube is unit-cell volumes,
so we get:
The substitution of integration over a sphere for the correct integral introduces another source of inaccuracy into the model.
The energy integral becomes
Changing the integration variable to ,
To simplify the appearance of this expression, define the Debye temperature
Many references[2][3] describe the Debye temperature as merely shorthand for some constants and material-dependent variables. However, as shown below, is roughly equal to the phonon energy of the minimum wavelength mode, and so we can interpret the Debye temperature as the temperature at which the highest frequency mode (and hence all modes) are excited.
Continuing, we then have the specific internal energy:
where is the (third) Debye function.
Differentiating with respect to we get the dimensionless heat capacity:
These formulae treat the Debye model at all temperatures. The more elementary formulae given further down give the asymptotic behavior in the limit of low and high temperatures. As already mentioned, this behaviour is exact, in contrast to the intermediate behaviour. The essential reason for the exactness at low and high energies, respectively, is that the Debye model gives (i) the exact dispersion relation at low frequencies, and (ii) corresponds to the exact sum rule concerning the number of vibrations per frequency interval.
Debye's derivation
Actually, Debye derived his equation somewhat differently and more simply. Using the solid mechanics of a continuous medium, he found that the number of vibrational states with a frequency less than a particular value was asymptotic to
in which is the volume and is a factor which he calculated from elasticity coefficients and density. Combining this with the expected energy of a harmonic oscillator at temperature T (already used by Einstein in his model) would give an energy of
if the vibrational frequencies continued to infinity. This form gives the behavior which is correct at low temperatures. But Debye realized that there could not be more than vibrational states for N atoms. He made the assumption that in an atomic solid, the spectrum of frequencies of the vibrational states would continue to follow the above rule, up to a maximum frequency chosen so that the total number of states is :
Debye knew that this assumption was not really correct (the higher frequencies are more closely spaced than assumed), but it guarantees the proper behavior at high temperature (the Dulong-Petit law). The energy is then given by:
- where is .
where is the function later given the name of third-order Debye function.
Low temperature limit
The temperature of a Debye solid is said to be low if , leading to
This definite integral can be evaluated exactly:
In the low temperature limit, the limitations of the Debye model mentioned above do not apply, and it gives a correct relationship between (phononic) heat capacity, temperature, the elastic coefficients, and the volume per atom (the latter quantities being contained in the Debye temperature).
High temperature limit
The temperature of a Debye solid is said to be high if . Using if leads to
This is the Dulong-Petit law, and is fairly accurate although it does not take into account anharmonicity, which causes the heat capacity to rise further. The total heat capacity of the solid, if it is a conductor or semiconductor, may also contain a non-negligible contribution from the electrons.
Debye versus Einstein

So how closely do the Debye and Einstein models correspond to experiment? Surprisingly close, but Debye is correct at low temperatures whereas Einstein is not.
How different are the models? To answer that question one would naturally plot the two on the same set of axes... except one can't. Both the Einstein model and the Debye model provide a functional form for the heat capacity. They are models, and no model is without a scale. A scale relates the model to its real-world counterpart. One can see that the scale of the Einstein model, which is given by
is . And the scale of the Debye model is , the Debye temperature. Both are usually found by fitting the models to the experimental data. (The Debye temperature can theoretically be calculated from the speed of sound and crystal dimensions.) Because the two methods approach the problem from different directions and different geometries, Einstein and Debye scales are not the same, that is to say
which means that plotting them on the same set of axes makes no sense. They are two models of the same thing, but of different scales. If one defines Einstein temperature as
then one can say
and, to relate the two, we must seek the ratio
The Einstein solid is composed of single-frequency quantum harmonic oscillators, . That frequency, if it indeed existed, would be related to the speed of sound in the solid. If one imagines the propagation of sound as a sequence of atoms hitting one another, then it becomes obvious that the frequency of oscillation must correspond to the minimum wavelength sustainable by the atomic lattice, .
which makes the Einstein temperature
and the sought ratio is therefore
Now both models can be plotted on the same graph. Note that this ratio is the cube root of the ratio of the volume of one octant of a 3-dimensional sphere to the volume of the cube that contains it, which is just the correction factor used by Debye when approximating the energy integral above.
Debye temperature table
Even though the Debye model is not completely correct, it gives a good approximation for the low temperature heat capacity of insulating, crystalline solids where other contributions (such as highly mobile conduction electrons) are negligible. For metals, the electron contribution to the heat is proportional to , which at low temperatures dominates the Debye result for lattice vibrations. In this case, the Debye model can only be said to approximate the lattice contribution to the specific heat. The following table lists Debye temperatures for several substances:[4] (apart from the ice entry)
|
|
Extension to other quasi-particles
For other bosonic quasi-particles, e.g. for magnons (quantized spin waves) in ferromagnets instead of the phonons (quantized sound waves) one easily derives analogous results. In this case at low frequencies one has different dispersion relations, e.g. in the case of magnons, instead of for phonons (with . One also has different sum rules (e.g. ). As a consequence, in ferromagnets one gets a magnon contribution to the heat capacity, which dominates at sufficiently low temperatures the phonon contribution In metals, in contrast, the main low-temperature contribution to the heat capacity, comes from the electrons. It is fermionic, and is calculated by different methods going back to Arnold Sommerfeld.
See also
References
- ^ 'Zur Theorie der spezifischen Waerme', Annalen der Physik (Leipzig) 39(4), p. 789 (1912)
- ^ Kittel, Charles, "Introduction to Solid State Physics", 7th Ed., Wiley, (1996)
- ^ Schroeder, Daniel V. "An Introduction to Thermal Physics" Addison-Wesley, San Francisco, Calif. (2000). Section 7.5
- ^ Kittel, Charles, Introduction to Solid State Physics, 7th Ed., Wiley, (1996)
- CRC Handbook of Chemistry and Physics, 56th Edition (1975–1976)
- Schroeder, Daniel V. An Introduction to Thermal Physics. Addison-Wesley, San Francisco, Calif. (2000). Section 7.5.
















![{\displaystyle {\sqrt[{3}]{N}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2632439311fdaac0db5c94be22a66bc4759c3b3e)
![{\displaystyle L/{\sqrt[{3}]{N}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/68ce3128288fa487eb59b377703ca3c31b6ac01d)
![{\displaystyle \lambda _{\rm {min}}={2L \over {\sqrt[{3}]{N}}}\,,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/67ce39568ba1619356396b79fafe20ab2a5b958f)
![{\displaystyle n_{\rm {max}}={\sqrt[{3}]{N}}\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/52e26b34255058c420ca488f519532157f051097)
![{\displaystyle U=\sum _{n_{x}}^{\sqrt[{3}]{N}}\sum _{n_{y}}^{\sqrt[{3}]{N}}\sum _{n_{z}}^{\sqrt[{3}]{N}}E_{n}\,{\bar {N}}(E_{n})\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fec6d96639ee52ea7d4e59bc75e719fe094fd5ce)
![{\displaystyle U\approx \int _{0}^{\sqrt[{3}]{N}}\int _{0}^{\sqrt[{3}]{N}}\int _{0}^{\sqrt[{3}]{N}}E(n)\,{\bar {N}}\left(E(n)\right)\,dn_{x}\,dn_{y}\,dn_{z}\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4f378b750c04cde69b47b010798779902e74825b)








![{\displaystyle U=\int _{0}^{\sqrt[{3}]{N}}\int _{0}^{\sqrt[{3}]{N}}\int _{0}^{\sqrt[{3}]{N}}E(n)\,{3 \over e^{E(n)/kT}-1}\,dn_{x}\,dn_{y}\,dn_{z}\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2b05660b876347cc0556fce9c94b050d78867006)




![{\displaystyle R={\sqrt[{3}]{6N \over \pi }}\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3f9d4e2bc889e4df97cb5eb25a732323e6d7dbd1)




![{\displaystyle T_{D}\ {\stackrel {\mathrm {def} }{=}}\ {hc_{s}R \over 2Lk}={hc_{s} \over 2Lk}{\sqrt[{3}]{6N \over \pi }}={hc_{s} \over 2k}{\sqrt[{3}]{{6 \over \pi }{N \over V}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/411d5aa9ab5603b7ea4354a1a15d081145b5d11d)




































![{\displaystyle \nu ={c_{s} \over \lambda }={c_{s}{\sqrt[{3}]{N}} \over 2L}={c_{s} \over 2}{\sqrt[{3}]{N \over V}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5d536a428a6732711cef27c5c9d7595d3db721b6)
![{\displaystyle T_{E}={\epsilon \over k}={h\nu \over k}={hc_{s} \over 2k}{\sqrt[{3}]{N \over V}}\,,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9192046046386dc246d52544f25f336af27e05bc)
![{\displaystyle {T_{E} \over T_{D}}={\sqrt[{3}]{\pi \over 6}}\,.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0f66884e3a913ba50ff2993657031c4028888ce1)






