Tonicity: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 71.125.75.76 to version by 67.49.232.86. False positive? Report it. Thanks, ClueBot NG. (225512) (Bot) |
|||
| Line 12: | Line 12: | ||
===Hypotonicity=== |
===Hypotonicity=== |
||
A hypotonic solution is a solution having a lesser solute concentration than the [[cytosol]]. It contains a lesser concentration of impermeable solutes on the external side of the membrane. When a cell’s cytoplasm is bathed in a hypotonic solution the water will be drawn out of the solution and into the cell by osmosis. If water molecules continue to diffuse into the cell, it will cause the cell to swell, up to the point that [[cytolysis]] (rupture) may occur. In plant cells, the cell will not always rupture. When placed in a hypotonic solution, the cell will have |
A hypotonic solution is a solution having a lesser solute concentration than the [[cytosol]]. It contains a lesser concentration of impermeable solutes on the external side of the membrane. When a cell’s cytoplasm is bathed in a hypotonic solution the water will be drawn out of the solution and into the cell by osmosis. If water molecules continue to diffuse into the cell, it will cause the cell to swell, up to the point that [[cytolysis]] (rupture) may occur. In plant cells, the cell will not always rupture. When placed in a hypotonic solution, the cell will have [[Turgor Pressure]] and proceed with its normal functions. |
||
===Isotonicity=== |
===Isotonicity=== |
||
Revision as of 21:46, 26 January 2011
Tonicity is a measure of the osmotic pressure (as defined by the water potential of the two solutions) of two solutions separated by a semipermeable membrane. It is commonly used when describing the response of cells immersed in an external solution. Like osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always be in equal concentrations on both sides of the membrane.
Osmotic pressure is the pressure that must be applied to a solution to prevent the inward flow of water across a semipermeable membrane.
Classification
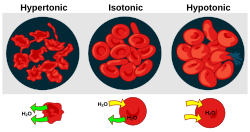
There are three classifications of tonicness that one solution can have relative to another. The three are hypertonic, hypotonic, and isotonic.
Hypertonicity
A hypertonic solution is a solution having a greater solute concentration than the cytosol. It contains a greater concentration of impermeable solutes on the external side of the membrane. When a cell’s cytoplasm is bathed in a hypertonic solution the water will be drawn into the solution and out of the cell by osmosis. If water molecules continue to diffuse out of the cell, it will cause the cell to shrink, or crenate.
Hypotonicity
A hypotonic solution is a solution having a lesser solute concentration than the cytosol. It contains a lesser concentration of impermeable solutes on the external side of the membrane. When a cell’s cytoplasm is bathed in a hypotonic solution the water will be drawn out of the solution and into the cell by osmosis. If water molecules continue to diffuse into the cell, it will cause the cell to swell, up to the point that cytolysis (rupture) may occur. In plant cells, the cell will not always rupture. When placed in a hypotonic solution, the cell will have Turgor Pressure and proceed with its normal functions.
Isotonicity
A condition or property of a solution in which its solute concentration is the same as the solute concentration of another solution with which it is compared.
Effect on cells
In eukaryotic animal cells, a hypertonic environment forces water to leave the cell so that the shape of the cell becomes distorted and wrinkled, a state known as crenation. In plant cells, the effect is more dramatic. The flexible cell membrane pulls away from the rigid cell wall, but remains joined to the cell wall at points called plasmodesmata. The cell takes on the appearance of a pincushion, and the plasmodesmata almost cease to function because they become constricted — a condition known as plasmolysis. In plant cells the terms isotonic, hypotonic and hypertonic cannot strictly be used accurately because the pressure exerted by the cell wall significantly affects the osmotic equilibrium point.
Some organisms have evolved intricate methods of circumventing hypertonicity. For example, saltwater is hypertonic to the fish that live in it. They need a large surface area in their gills in contact with seawater for gas exchange, thus they lose water osmotically to the sea from gill cells. They respond to the loss by drinking large amounts of saltwater, and actively excreting the excess salt. This process is called osmoregulation.
In a hypotonic environment, animal cells will swell until they burst, a process known as cytolysis. Fresh water fish urinate constantly to prevent cytolysis. Plant cells tend to resist bursting, due to the reinforcement of their cell wall, which provides effective osmolarity or osmolality.
In some cases of suspensions intended for intramuscular injection, a slightly hypotonic solution is preferred in order to increase the dissolution and absorption of the drug by absorbing water from the surrounding tissues.
