Tetramethylbutane: Difference between revisions
Appearance
Content deleted Content added
Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ( |
No edit summary |
||
| Line 32: | Line 32: | ||
The compound can be obtained by reaction of [[Grignard reagent]] [[tert-Butylmagnesium bromide|''tert''-butylmagnesium bromide]] with [[ethyl bromide]], or of [[ethylmagnesium bromide]] with [[tert-butyl bromide|''tert''-butyl bromide]] in the presence of [[manganese]]([[Valence (chemistry)|II]]) ions.<ref name="KHARASCH">{{cite journal | author = M. S. KHARASCH, J. W. HANCOCK, W. NUDENBERG, P. O. TAWNEY | year = 1956 | title = Factors Influencing the Course and Mechanism of Grignard Reactions. XXII. The Reaction of Grignard Reagents with Alkyl Halides and Ketones in the Presence of Manganous Salts | journal = Journal of Organic Chemistry | volume = 21 | issue = 3 | pages = 322–327 | doi=10.1021/jo01109a016}}</ref> |
The compound can be obtained by reaction of [[Grignard reagent]] [[tert-Butylmagnesium bromide|''tert''-butylmagnesium bromide]] with [[ethyl bromide]], or of [[ethylmagnesium bromide]] with [[tert-butyl bromide|''tert''-butyl bromide]] in the presence of [[manganese]]([[Valence (chemistry)|II]]) ions.<ref name="KHARASCH">{{cite journal | author = M. S. KHARASCH, J. W. HANCOCK, W. NUDENBERG, P. O. TAWNEY | year = 1956 | title = Factors Influencing the Course and Mechanism of Grignard Reactions. XXII. The Reaction of Grignard Reagents with Alkyl Halides and Ketones in the Presence of Manganous Salts | journal = Journal of Organic Chemistry | volume = 21 | issue = 3 | pages = 322–327 | doi=10.1021/jo01109a016}}</ref> |
||
The full IUPAC name of the compound is '''2,2,3,3-tetramethylbutane''', but the numbers are superfluous in this case because there is no other kind of "tetramethylbutane". |
The full IUPAC name of the compound is '''2,2,3,3-tetramethylbutane''', but the numbers are superfluous in this case because there is no other possible kind of "tetramethylbutane". |
||
==See also== |
==See also== |
||
Revision as of 08:30, 27 July 2011
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,2,3,3-Tetramethylbutane
| |||
| Other names
Hexamethylethane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.008.961 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C8H18 | |||
| Molar mass | 114.232 g·mol−1 | ||
| Melting point | 100.8 °C[2] | ||
| Boiling point | 107 °C (1020 mbar)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
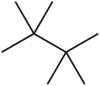
Tetramethylbutane, sometimes called hexamethylethane, is a hydrocarbon with formula C8H18 or (H3C-)3C-C(-CH3)3. It is the most heavily branched and most compact of the many octane isomers, the only one with a butane (C4) backbone.
The compound can be obtained by reaction of Grignard reagent tert-butylmagnesium bromide with ethyl bromide, or of ethylmagnesium bromide with tert-butyl bromide in the presence of manganese(II) ions.[3]
The full IUPAC name of the compound is 2,2,3,3-tetramethylbutane, but the numbers are superfluous in this case because there is no other possible kind of "tetramethylbutane".
See also
References
- ^ 2,2,3,3-Tetramethylbutane at Sigma-Aldrich
- ^ a b Scott, D.W.; Douslin, D.R.; Gross, M.E.; Oliver, G.D.; Huffman, H.M.: 2,2,3,3-Tetramethylbutane: Heat capacity, heats of transition, fusion and sublimation, vapor pressure, entropy and thermodynamic functions in J. Am. Chem. Soc. 74 (1952) 883-887.
- ^ M. S. KHARASCH, J. W. HANCOCK, W. NUDENBERG, P. O. TAWNEY (1956). "Factors Influencing the Course and Mechanism of Grignard Reactions. XXII. The Reaction of Grignard Reagents with Alkyl Halides and Ketones in the Presence of Manganous Salts". Journal of Organic Chemistry. 21 (3): 322–327. doi:10.1021/jo01109a016.
{{cite journal}}: CS1 maint: multiple names: authors list (link)