Chytridiomycosis: Difference between revisions
5e ext2013 (talk | contribs) No edit summary |
5e ext2013 (talk | contribs) No edit summary |
||
| Line 36: | Line 36: | ||
− Experiments were done on a variety of different species with a variety of different approaches trying to discover a viable treatment option. The first experiment was conducted on metamorphosed Bufo bufo with antimicrobial peptides on five separate groups under different conditions. Though it was determined that the peptide treatment did not increase survival rate overall, one particular group did show some signs that the idea shouldn’t be abandoned. This group of three was treated with the peptide first and then exposed to Batrachochytrium dendrobatidis and upon retesting at the end of the study, all three tested negative for Bd. <ref>{{cite journal|last=Woodhams|first=D.C.|coauthors=Reinert, L. K., Rollins-Smith, L., Lam, B., Harris, R. N., Voyles, J.|title=Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy|journal=Diseases of Aguatic Organisms|year=2012|volume=98|issue=1|pages=11-25|accessdate=24 October 2013}}</ref> |
− Experiments were done on a variety of different species with a variety of different approaches trying to discover a viable treatment option. The first experiment was conducted on metamorphosed Bufo bufo with antimicrobial peptides on five separate groups under different conditions. Though it was determined that the peptide treatment did not increase survival rate overall, one particular group did show some signs that the idea shouldn’t be abandoned. This group of three was treated with the peptide first and then exposed to Batrachochytrium dendrobatidis and upon retesting at the end of the study, all three tested negative for Bd. <ref>{{cite journal|last=Woodhams|first=D.C.|coauthors=Reinert, L. K., Rollins-Smith, L., Lam, B., Harris, R. N., Voyles, J.|title=Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy|journal=Diseases of Aguatic Organisms|year=2012|volume=98|issue=1|pages=11-25|accessdate=24 October 2013}}</ref> |
||
+ <ref name="Hooker 1981">{{cite book|author=Hooker WJ. |title=Compendium of Potato Diseases|url=http://books.google.com/books?id=h6HmE1MtcpoC&pg=PA36 |year=1981 |publisher=International Potato Center |isbn=978-0-89054-027-5 |pages=36–7}}</ref> |
|||
− Itraconazol was used in two studies. The first experiment was conducted on Rana muscosa that were extracted from Sixty Lake Basin in the Sierra Nevada Mountains of California, USA. These frogs were all in advanced stages of infection and itraconazol was deemed ineffective. However, it was effective in another study combining Itraconazol with heat (35 degrees C). Lithobates pipiens showed success with the combination of therapies with four of four previously infected frogs in two separate groups tested completely negative and only one of seven uninfected frogs became infected after treatment.Though these tests did not develop an exact treatment, they are instrumental in developing treatment protocols and materials that should continue to be tested in the future. <ref>Woodhams, D. C., Geiger, C. C., Reinert, L. K., Rollins-Smith, L., Lam, B., Harris, R. N., . . . Voyles, J. (2012). Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Diseases of Aquatic Organisms, 98(1), 11-25. doi:http://dx.doi.org/10.3354/dao02429 </ref> |
− Itraconazol was used in two studies. The first experiment was conducted on Rana muscosa that were extracted from Sixty Lake Basin in the Sierra Nevada Mountains of California, USA. These frogs were all in advanced stages of infection and itraconazol was deemed ineffective. However, it was effective in another study combining Itraconazol with heat (35 degrees C). Lithobates pipiens showed success with the combination of therapies with four of four previously infected frogs in two separate groups tested completely negative and only one of seven uninfected frogs became infected after treatment.Though these tests did not develop an exact treatment, they are instrumental in developing treatment protocols and materials that should continue to be tested in the future. <ref>Woodhams, D. C., Geiger, C. C., Reinert, L. K., Rollins-Smith, L., Lam, B., Harris, R. N., . . . Voyles, J. (2012). Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Diseases of Aquatic Organisms, 98(1), 11-25. doi:http://dx.doi.org/10.3354/dao02429 </ref> |
||
Revision as of 19:05, 24 October 2013

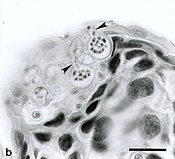
Chytridiomycosis is an infectious disease of amphibians, caused by the chytrid Batrachochytrium dendrobatidis, a non-hyphal zoosporic fungus. Chytridiomycosis has been linked to dramatic population declines or even extinctions of amphibian species in western North America, Central America, South America, eastern Australia, and Dominica and Montserrat in the Caribbean. The fungus is capable of causing sporadic deaths in some amphibian populations and 100% mortality in others. There is no effective measure for control of the disease in wild populations. The disease has been proposed as a contributing factor to a global decline in amphibian populations that apparently has affected 30% of the amphibian species of the world.[1]
History
The disease in its epizootic form was first discovered in 1993 in dead and dying frogs in Queensland, Australia. Research since then has shown that it had been present in the country since at least 1978 and is widespread across Australia. It is also found in Africa, the Americas, Europe, New Zealand and Oceania. In Australia, Panama, and New Zealand, the fungus seemed to have suddenly ‘appeared’ and expanded its range at the same time frog numbers declined. However, it may simply be that the fungus occurs naturally and was only identified recently because it has become more virulent or more prevalent in the environment, or because host populations have become less resistant to the disease. The fungus has been detected in four areas of Australia — the east coast, Adelaide, south-west Western Australia and the Kimberley — and is probably present elsewhere.[2]
The oldest documented case of Batrachochytrium is from a specimen of an African clawed frog (Xenopus laevis) collected in 1938, and this species also appears to be essentially unaffected by the disease, making it a suitable vector.[3] The first well-documented method of human pregnancy testing involved this species, and as a result large-scale international trade in living African clawed frogs began more than 60 years ago.[3] If Batrachochytrium originated in Africa, it has been theorized that the African clawed frog was the vector of the initial spread out of the continent.[3] The earliest documented case of the disease chytridiomycosis was an American bullfrog (Rana catesbeiana) collected in 1978.[3] It is still not clear if it is a new emergent pathogen or if it is an old pathogen with recently increased virulence.
Evolutionary History of Chytrid Fungus Batrachochytrium dendrobatidis
− − Understanding the evolutionary history of Emerging Infectious Diseases (EID's) is critical to understanding the origin and spread of EID's and focusing disease mitigating efforts. Studies sequencing the genome of Batrachochytrium dendrobatidis(Bd) partially answer a lot of important questions. 29 isolates for this study combined with 49 global isolates used in another study and then rooted with a non-Bd chytrid (Homolaphlyctis polyrhiza, Hp)allowed the group to infer the branching evolutionary event. The tree topologies indicate far greater genetic and evolutionary diversity in Bd then previous recognized. the study concludes that Bd is composed of multiple divergent lineages and that the emergence of the globally distributed GPL occurred within the context of deeper ancestral diversity, much of which may yet be discovered. [4]
− − Two hypotheses have been suggested to describe this pathogen: novel or endemic. This study suggests that it may not be one or the other but both. Some of the isolates did share a common geography and were closely related suggesting that this is not ancient geographic structure or long-term association but more likely, invasion and subsequent evolution of clones in a particular region. However, there are lineages present that diverged before the GPL (global panzootic lineage). GPL is a term used to describe similar, geographically widespread lineages. The fact that there are lineages present that diverged before the GPL suggests that Bd is far more ancient than represented by the novel pathogen hypothesis which suggests that Bd is endemic in some parts of the world. So it is very possible that Bd is both novel and endemic at the same time. [4]
Disease progression
Chytridiomycosis is believed to adhere to the following course: zoospores first encounter amphibian skin and quickly give rise to sporangia, which produce new zoospores.[5] The disease then progresses as these new zoospores reinfect the host. Morphological changes in amphibians infected with the fungus include a reddening of the ventral skin, convulsions with extension of hind limbs, accumulations of sloughed skin over the body, sloughing of the superficial epidermis of the feet and other areas, slight roughening of the surface with minute skin tags, and occasional small ulcers or hemorrhage. Behavioral changes can include lethargy, a failure to seek shelter, a failure to flee, a loss of righting reflex, and abnormal posture (e.g. sitting with the hind legs away from the body).[6]
Research
Laboratory studies suggest that the amphibian chytrid fungus grows best between 17-25°C,[7] and that exposure of infected frogs to high temperatures can cure the frogs.[8] In nature, the more time individual frogs were found at temperatures above 25°C, the less likely they were to be infected by the amphibian chytrid.[9] This may explain why chytridiomycosis-induced amphibian declines have occurred primarily at higher elevations and during cooler months.[10] It has been shown that naturally produced cutaneous peptides can inhibit the growth of B. dendrobatidis when the infected amphibians are around temperatures near 10 °C (50 °F), allowing species like the northern leopard frog (Rana pipiens) to clear the infection in about 15% of cases.[11]
Although many declines have been credited to the fungus B. dendrobatidis, there are species that resist the infection and some reports have found that some populations can survive with a low level of persistence of the disease.[12] In addition, some species that seem to resist the infection may actually harbor a non-pathogenic form of Batrachochytrium dendrobatidis.
Some researchers contend that the focus on chytridiomycosis has made amphibian conservation efforts dangerously myopic. A review of the data in the IUCN Red List found that the threat of the disease was assumed in most cases, but that there was no evidence that it is, in fact, a threat.[13] Conservation efforts in New Zealand continue to be focused on curing the critically endangered native Archey's frog, Leiopelma archeyi, of chytridiomycosis even though research has shown clearly that they are immune from infection by B. dendrobatidis and are dying in the wild of other still-to-be identified diseases.[14] In Guatemala, several thousands of tadpoles perished from an unidentified pathogen distinct from B. dendrobatidis.[15] Such researchers stress the need for a broader understanding of the host-parasite ecology that is contributing to the modern day amphibian declines.
Treatment options
Reid Harris of James Madison University has found that coating frogs with Janthinobacterium lividum appears to protect them from chytridiomycosis.[16]
Archey's frog, Leiopelma archeyi, was successfully cured of chytridiomycosis by applying chloramphenicol topically.[17]
− Experiments were done on a variety of different species with a variety of different approaches trying to discover a viable treatment option. The first experiment was conducted on metamorphosed Bufo bufo with antimicrobial peptides on five separate groups under different conditions. Though it was determined that the peptide treatment did not increase survival rate overall, one particular group did show some signs that the idea shouldn’t be abandoned. This group of three was treated with the peptide first and then exposed to Batrachochytrium dendrobatidis and upon retesting at the end of the study, all three tested negative for Bd. [18]
− Itraconazol was used in two studies. The first experiment was conducted on Rana muscosa that were extracted from Sixty Lake Basin in the Sierra Nevada Mountains of California, USA. These frogs were all in advanced stages of infection and itraconazol was deemed ineffective. However, it was effective in another study combining Itraconazol with heat (35 degrees C). Lithobates pipiens showed success with the combination of therapies with four of four previously infected frogs in two separate groups tested completely negative and only one of seven uninfected frogs became infected after treatment.Though these tests did not develop an exact treatment, they are instrumental in developing treatment protocols and materials that should continue to be tested in the future. [19]
See also
References
- ^ Stuart, S. N., J. S. Chanson, et al. (2004). "Status and trends of amphibian declines and extinctions worldwide." Science 306: 1783-1786.
- ^ "Chytridiomycosis (Amphibian Chytrid Fungus Disease)" (PDF). Australian Government Department of Sustainability, Environment, Water, Population and Communities. Retrieved 14 October 2013.
- ^ a b c d Weldon; du Preez; Hyatt; Muller; and Speare (2004). Origin of the Amphibian Chytrid Fungus. Emerging Infectious Disease 10(12).
- ^ a b Rosenblum, E. E. (n.d). Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. 110(23), 9385-9391.
- ^ Berger, L., Hyatt, A.D., Speare, R. & Longcore, J.E. Dis. Aquat. Org. 68, 51-63 (2005).
- ^ Padgett-Flohr, G.E. (2007). "Amphibian Chytridiomycosis: An Informational Brochure" (PDF). California Center for Amphibian Disease Control. Retrieved 14 October 2013.
- ^ Piotrowski, J. S., Annis, S. L. & Longcore, J. E. (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9–15
- ^ Woodhams, D. C., R. A. Alford, et al. (2003). "Emerging disease of amphibians cured by elevated body temperature." Diseases of aquatic organisms 55: 65-67.
- ^ Rowley, J.J.L. & Alford, R.A. (2013) Hot bodies protect amphibians against chytrid infection in nature. Scientific Reports 3, 1515. doi:10.1038/srep01515
- ^ Woodhams, D. C. & Alford, R. A. (2005) The ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 19, 1449–1459
- ^ Voordouw, M. J., D. Adama, B. Houston, and P. Govindarajulu. "Prevalence of the Pathogenic Chytrid Fungus, Batrachochytrium Dendrobatidis, in an Endangered Population of Northern Leopard Frogs, Rana Pipiens." BMC Ecol. 6th ser. 10.1 (2010). Print.
- ^ Retallick, R. W. R., H. McCallum, et al. (2004). "Endemic Infection of the Amphibian Chytrid Fungus in a Frog Community Post-Decline." PLoS Biology 2(11): e351.
- ^ Heard M, Smith KF, Ripp K, 2011 Examining the Evidence for Chytridiomycosis in Threatened Amphibian Species. PLoS ONE 6(8): e23150. doi:10.1371/journal.pone.0023150
- ^ Waldman B (2011) Brief encounters with Archey's Frog. FrogLog 99:39-41.
- ^ Di Rosa, Ines et al. "The Proximate Cause of Frog Declines?" Nature 447.31 (2007) E4-E5.
- ^ Probiotic bug is a frog lifesaver, Linda Geddes, New Scientist, Issue 2711, June 8, 2009
- ^ Elimination of the amphibian chytrid fungus Batrachochytrium dendrobatidis by Archey’s frog Leiopelma archeyi, Phillip J. Bishop, Diseases of Aquatic Organisms, Vol. 84: 9–15, 2009 doi:10.3354/dao02028
- ^ Woodhams, D.C. (2012). "Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy". Diseases of Aguatic Organisms. 98 (1): 11–25.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Woodhams, D. C., Geiger, C. C., Reinert, L. K., Rollins-Smith, L., Lam, B., Harris, R. N., . . . Voyles, J. (2012). Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Diseases of Aquatic Organisms, 98(1), 11-25. doi:http://dx.doi.org/10.3354/dao02429
