Mancozeb: Difference between revisions
add image |
Referring to Cornell page on toxicity. |
||
| Line 6: | Line 6: | ||
Mancozeb reacts with, and inactivates, the [[sulfhydryl group]]s of [[amino acid]]s and [[enzyme]]s within fungal cells, resulting in disruption of [[lipid metabolism]], respiration, and production of [[adenosine triphosphate]].<ref>{{Cite book | title = The Pesticide Manual - A world compendium | edition = Thirteenth | author = Tomlin C.D.S | publisher = British Crop Protection Council | date = 2003}}</ref> |
Mancozeb reacts with, and inactivates, the [[sulfhydryl group]]s of [[amino acid]]s and [[enzyme]]s within fungal cells, resulting in disruption of [[lipid metabolism]], respiration, and production of [[adenosine triphosphate]].<ref>{{Cite book | title = The Pesticide Manual - A world compendium | edition = Thirteenth | author = Tomlin C.D.S | publisher = British Crop Protection Council | date = 2003}}</ref> |
||
Mancozeb is listed under [[Fungicide Resistance Action Committee|FRAC]] code M:03 The "M:" refers to Chemicals with Multi-Site Activity. "M:" FRAC groups are defined as generally considered as a low risk group without any signs of resistance developing to the fungicides.<ref>{{cite web|title=FRAC Code List ©*2017 |url=http://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2017-final.pdf?sfvrsn=fab94a9a_2 |publisher=Fungicide Resistance Action Committee |accessdate=November 27, 2017}}</ref> |
Mancozeb is listed under [[Fungicide Resistance Action Committee|FRAC]] code M:03 The "M:" refers to Chemicals with Multi-Site Activity. "M:" FRAC groups are defined as generally considered as a low risk group without any signs of resistance developing to the fungicides.<ref>{{cite web|title=FRAC Code List ©*2017 |url=http://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2017-final.pdf?sfvrsn=fab94a9a_2 |publisher=Fungicide Resistance Action Committee |accessdate=November 27, 2017}}</ref>. A major toxicological concern, however, is ethylenethiourea (ETU), an industrial contaminant and a breakdown product of mancozeb and other EBDC pesticides. In addition to having the potential to cause goiter, a condition in which the thyroid gland is enlarged, this metabolite has produced birth defects and cancer in experimental animals. ETU has been classified as a probable human carcinogen by the EPA.<ref>http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/mancozeb-ext.html</ref> |
||
==See also== |
==See also== |
||
Revision as of 17:50, 20 July 2019
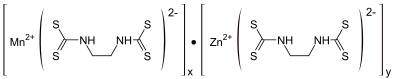
Mancozeb is a dithiocarbamate non-systemic agricultural fungicide with multi-site, protective action on contact. It is a combination of two other dithiocarbamates: maneb and zineb.[1] The mixture controls many fungal diseases in a wide range of field crops, fruits, nuts, vegetables, and ornamentals. It is marketed as Penncozeb, Trimanoc, Vondozeb, Dithane, Manzeb, Nemispot, and Manzane. In Canada, a mixture of zoxamide and mancozeb was registered for control of the mildew named Gavel as early as 2008.[2]
Mechanism
Mancozeb reacts with, and inactivates, the sulfhydryl groups of amino acids and enzymes within fungal cells, resulting in disruption of lipid metabolism, respiration, and production of adenosine triphosphate.[3]
Mancozeb is listed under FRAC code M:03 The "M:" refers to Chemicals with Multi-Site Activity. "M:" FRAC groups are defined as generally considered as a low risk group without any signs of resistance developing to the fungicides.[4]. A major toxicological concern, however, is ethylenethiourea (ETU), an industrial contaminant and a breakdown product of mancozeb and other EBDC pesticides. In addition to having the potential to cause goiter, a condition in which the thyroid gland is enlarged, this metabolite has produced birth defects and cancer in experimental animals. ETU has been classified as a probable human carcinogen by the EPA.[5]
See also
References
- ^ "Mancozeb". Cornell University. 1993. Retrieved 2014-07-20.
It is a combination of two other chemicals of this class, maneb and zineb
- ^ "Gowan buys Dow's Gavel potato fungicide". grainews.ca. July 18, 2008.
- ^ Tomlin C.D.S (2003). The Pesticide Manual - A world compendium (Thirteenth ed.). British Crop Protection Council.
- ^ "FRAC Code List ©*2017" (PDF). Fungicide Resistance Action Committee. Retrieved November 27, 2017.
- ^ http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/mancozeb-ext.html
External links
- Mancozeb in the Pesticide Properties DataBase (PPDB)
