Hexon protein: Difference between revisions
Corrected incorrect link to irrelevant lemma |
Linked relevant proteins, removed irrelevant Exxon link, and removed incorrect time reference. |
||
| Line 1: | Line 1: | ||
{{distinguish|text=[[exon]], a part of a gene |
{{distinguish||text=[[exon]], a part of a gene, or [[axon]], the projection of a [[neuron]]}} |
||
{{Infobox protein family |
{{Infobox protein family |
||
| Line 41: | Line 41: | ||
In molecular biology, the '''hexon protein''' is a major coat [[protein]] found in [[Adenoviruses]]. Hexon coat [[protein]]s are synthesised during late [[infection]] and form homo-trimers. The 240 copies of the hexon [[Trimer (biochemistry)|trimer]] that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of [[polypeptide]] IX. The penton complex, formed by the peripentonal hexons and penton base (holding in place a fibre), lie at each of the 12 vertices.<ref name="pmid7932702">{{cite journal |vauthors=Athappilly FK, Murali R, Rux JJ, Cai Z, Burnett RM | title = The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution | journal = J. Mol. Biol. | volume = 242 | issue = 4 | pages = 430–55 |date=September 1994 | pmid = 7932702 | doi = 10.1006/jmbi.1994.1593| url = }}</ref> The hexon coat protein is a duplication consisting of two [[protein domain|domains]] with a similar [[protein folding|fold]] packed together like the nucleoplasmin [[protein subunit|subunits]]. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The [[Protein domain|domains]] have a beta-sandwich [[secondary structure|structure]] consisting of 8 strands in two [[beta sheet|sheets]] with a jelly-roll topology; each domain is heavily decorated with many insertions.<ref name="pmid12915569">{{cite journal |vauthors=Rux JJ, Kuser PR, Burnett RM | title = Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods | journal = J. Virol. | volume = 77 | issue = 17 | pages = 9553–66 |date=September 2003 | pmid = 12915569 | pmc = 187380 | doi = 10.1128/jvi.77.17.9553-9566.2003| url = }}</ref> Some hexon proteins contain a distinct C-terminal domain. |
In molecular biology, the '''hexon protein''' is a major coat [[protein]] found in [[Adenoviruses]]. Hexon coat [[protein]]s are synthesised during late [[infection]] and form homo-trimers. The 240 copies of the hexon [[Trimer (biochemistry)|trimer]] that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of [[polypeptide]] IX. The penton complex, formed by the peripentonal hexons and penton base (holding in place a fibre), lie at each of the 12 vertices.<ref name="pmid7932702">{{cite journal |vauthors=Athappilly FK, Murali R, Rux JJ, Cai Z, Burnett RM | title = The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution | journal = J. Mol. Biol. | volume = 242 | issue = 4 | pages = 430–55 |date=September 1994 | pmid = 7932702 | doi = 10.1006/jmbi.1994.1593| url = }}</ref> The hexon coat protein is a duplication consisting of two [[protein domain|domains]] with a similar [[protein folding|fold]] packed together like the nucleoplasmin [[protein subunit|subunits]]. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The [[Protein domain|domains]] have a beta-sandwich [[secondary structure|structure]] consisting of 8 strands in two [[beta sheet|sheets]] with a jelly-roll topology; each domain is heavily decorated with many insertions.<ref name="pmid12915569">{{cite journal |vauthors=Rux JJ, Kuser PR, Burnett RM | title = Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods | journal = J. Virol. | volume = 77 | issue = 17 | pages = 9553–66 |date=September 2003 | pmid = 12915569 | pmc = 187380 | doi = 10.1128/jvi.77.17.9553-9566.2003| url = }}</ref> Some hexon proteins contain a distinct C-terminal domain. |
||
In a |
In a paper (Bremner et al., 2009) it was shown that hexon directly recruits the cellular motor protein [[dynein]] in a pH-dependent manner. The dynein-regulatory protein, [[dynactin]], was found to play a clear role in regulating the dynein-adenovirus complex transport to the nucleus.<ref>{{cite journal | title = Adenovirus Transport via Direct Interaction of Cytoplasmic Dynein with the Viral Capsid Hexon Subunit |doi=10.1016/j.chom.2009.11.006|pmc=2810746}}</ref> |
||
==References== |
==References== |
||
Revision as of 21:54, 4 July 2020
| Adeno_hexon | |||||||||
|---|---|---|---|---|---|---|---|---|---|
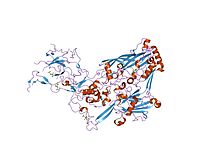 refinement of adenovirus type 2 hexon with cns | |||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon | ||||||||
| Pfam | PF01065 | ||||||||
| InterPro | IPR016107 | ||||||||
| SCOP2 | 1dhx / SCOPe / SUPFAM | ||||||||
| |||||||||
| Adeno_hexon_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 the quasi-atomic model of human adenovirus type 5 capsid (part 2) | |||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon_C | ||||||||
| Pfam | PF03678 | ||||||||
| InterPro | IPR016108 | ||||||||
| SCOP2 | 1dhx / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology, the hexon protein is a major coat protein found in Adenoviruses. Hexon coat proteins are synthesised during late infection and form homo-trimers. The 240 copies of the hexon trimer that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of polypeptide IX. The penton complex, formed by the peripentonal hexons and penton base (holding in place a fibre), lie at each of the 12 vertices.[1] The hexon coat protein is a duplication consisting of two domains with a similar fold packed together like the nucleoplasmin subunits. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The domains have a beta-sandwich structure consisting of 8 strands in two sheets with a jelly-roll topology; each domain is heavily decorated with many insertions.[2] Some hexon proteins contain a distinct C-terminal domain.
In a paper (Bremner et al., 2009) it was shown that hexon directly recruits the cellular motor protein dynein in a pH-dependent manner. The dynein-regulatory protein, dynactin, was found to play a clear role in regulating the dynein-adenovirus complex transport to the nucleus.[3]
References
- ^ Athappilly FK, Murali R, Rux JJ, Cai Z, Burnett RM (September 1994). "The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution". J. Mol. Biol. 242 (4): 430–55. doi:10.1006/jmbi.1994.1593. PMID 7932702.
- ^ Rux JJ, Kuser PR, Burnett RM (September 2003). "Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods". J. Virol. 77 (17): 9553–66. doi:10.1128/jvi.77.17.9553-9566.2003. PMC 187380. PMID 12915569.
- ^ "Adenovirus Transport via Direct Interaction of Cytoplasmic Dynein with the Viral Capsid Hexon Subunit". doi:10.1016/j.chom.2009.11.006. PMC 2810746.
{{cite journal}}: Cite journal requires|journal=(help)
