Dictyostelium discoideum
| Dictyostelium discoideum | |
|---|---|

| |
| Fruiting bodies of D. discoideum | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | Dictyostelia
|
| Order: | Dictyosteliida
|
| Family: | Dictyosteliidae
|
| Genus: | |
| Species: | D. discoideum
|
Dictyostelium discoideum is a species of soil-living amoeba belonging to the group Mycetozoa. It is a primitive eukaryote that has been used to study the mechanisms of cell movement, chemotaxis, and cell signaling, as well as the genes involved in cellular differentiation and pattern formation. Its simplicity and resistance to damage allow the development and behaviors of D. discoideum to be examined at the cellular, biochemical, and genetic levels. D. discoideum has a simple life cycle that includes vegetative, aggregation, migration, and culmination stages. This life cycle of D. discoideum, as with all dictyostelids, is notable because of the transition from an early unicellular existence to a later multicellular slug-like pseudoplasmodium. These characteristics make it a valuable model for studying a variety of developmental, genetic, and physiological processes in many other organisms.
Natural habitat and diet
In the wild, D. discoideum can be found in soil and moist leaf litter. The primary natural diet of D. discoideum consists of bacteria that are found in the soil and decaying organic matter such as Escherichia coli. Uninucleate amoebae of D. discoideum consumes bacteria contiguous to its natural habitat, which includes deciduous forest soil and decaying leaves.[1]
Life cycle and reproduction
The life cycle of D. discoideum begins as spores are released from a mature fruiting body. Myxamoebae hatch from the spores under warm and moist conditions. The myxamoebae divide by mitosis as they feed on bacteria. This is known as the vegetative stage. The bacteria secrete folic acid, attracting the myxamoebae. When the supply of bacteria is depleted, the myxamoebae enter into the aggregation stage.
During aggregation, starvation initiates the creation of a biochemical machinery.[2] This machinery includes glycoproteins and adenylyl cyclase. The glycoproteins will be used for cell-cell adhesion in the membrane and adenylyl cyclase creates cyclic AMP, a very important factor in this stage of development. Cyclic AMP is secreted by the central amoebae and all the others move towards it. As the amoebas move, they bump into each other and stick together as glycoprotein adhesion molecules.

In the migration stage, the aggregated amoebae form an elongated mound that tips over to form the pseudoplasmodium, or slug. The slug is approximately 2-4 mm long and is capable of movement by producing a cellulose sheath in its anterior cells through which the slug moves.[3] Part of this sheath gets left behind the pseudoplasmodium as it moves toward light, heat, and humidity, creating a slimy trail.[3] It has an anterior and posterior end and moves only in a forward direction. The pseudoplasmodium already has differentiated cell types.[3] The anterior end of the pseudoplasmodium will form the stalk of the fruiting body and is made up of a type of cell called prestalk cells.[3] The posterior end will form the spores of the fruiting body and are made up of cells called prespore cells.[3] Anterior-like cells, which have only been recently discovered, are also dispersed throughout the posterior region of the slug, and they form the very bottom of the fruiting body, as well as the caps of the spores.[3] Cyclic AMP, as well as a substance called differentiation-inducing factor (DIF), help to form these different cell types.[3] After the slug settles into one spot, the posterior end spreads out with the anterior end raised in the air, forming what is called the "Mexican hat", and beginning the culmination stage.
The prestalk cells and prespore cells switch positions in the culmination stage in order to form the mature fruiting body.[3] The anterior end of the Mexican hat forms a cellulose tube which allows the more posterior cells to move up the outside of the tube to the top, and the prestalk cells move down to where they need to be.[3] This rearrangement forms the stalk of the fruiting body made up of the cells from the anterior end of the slug, and the cells from the posterior end of the slug are on the top and now form the spores of the fruiting body. At the end of this 8-10 hours process, the mature fruiting body is fully formed.[3] This fruiting body is about 1-2 mm tall and is now able to start the entire cycle over again by releasing the mature spores that become myxamoebae.
Generally reproducing asexually, D. discoideum are still capable of sexual reproduction if certain conditions are met. If two amoebae of different mating types are present in a dark and wet environment, they can fuse during aggregation to form a giant cell. The giant cell will then engulf the other cells in the aggregate and encase the whole aggregate in a thick, cellulose wall to protect it. This is known as a macrocyst. Inside the macrocyst, the giant cell divides first through meiosis, then through mitosis to produce many haploid amoebae that will be released to feed as normal amoebae would. While sexual reproduction is possible, it is very rare and scientists have yet to see successful germination of a D. discoideum macrocyst under laboratory conditions.
Use as a model organism
Because of the simplicity of the life cycle of D. discoideum, it is commonly used as a model organism. They can be observed at organismic, cellular, and molecular levels primarily because of their restricted number of cell types, behaviors, and their rapid growth (Tyler 2006). Cell differentiation is the process that occurs when a cell becomes more specialized to become a multicellular organism (Cellular Differentiation” 2008). Changes in size, shape, metabolic activities, and responsiveness can occur as a result of adjustments in gene expression (Cellular Differentiation 2008). Cell diversity and differentiation, in this species, involves decisions made from cell-cell interactions in pathways to either stalk cells or spore cells (Kay et al. 1978). These cell fates depend on their environment and pattern formation. Therefore, the organism is an excellent model for studying cell differentiation.
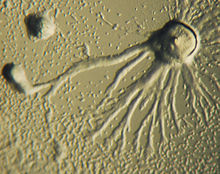
Chemotaxis is defined as a passage of an organism toward or away from a chemical stimulus along a chemical concentration gradient (The American Heritage 2008). Certain organisms demonstrate chemotaxis when they move toward a supply of nutrients (Wordnet 2008). In D. discoideum, the amoeba secretes the signal, cAMP, out of the cell attracting other amoebas to migrate toward the source. Every amoeba moves toward a central amoeba, the one dispensing the greatest amount of cAMP secretions. The secretion of the cAMP is then exhibited by all amoebas and is a call for the amoebas to begin aggregation. These chemical emissions and amoeba movement occur every six minutes. The amoebas move toward the concentration gradient for sixty seconds and stop until the next secretion is sent out. The use of cAMP as a chemotactic agent is not established in any other organism. In developmental biology, this is one of the comprehensible examples of chemotaxis.[3]
Programmed cell death, also referred to as apoptosis, is a normal part of species development.[2] Apoptosis is necessary for the proper spacing and sculpting of complex organs. D. discoideum uses apoptosis in the formation of the mature fruiting body. During the pseudoplasmodium (slug or grex) stage of its life cycle, the organism has formed three main types of cells: prestalk, prespore, and anterior-like cells. The prestalk cells, during culmination, secrete a cellulose coat and extend as a tube through the grex.[2] As they differentiate, they form vacuoles and enlarge lifting up the prespore cells. The stalk cells undergo apoptosis and die as the prespore cells are lifted high above the substrate. The prespore cells then become spore cells; each one becoming a new myxamoeba upon dispersal (Tyler 2006). This is an example of how apoptosis is used in the formation of a reproductive organ, the mature fruiting body. Cell differentiation, chemotaxis, and programmed cell death are all reasons why D. discoideum has and is still being used as a model organism for scientists to study.
Legionella is a genus of bacteria that includes species that can cause Legionnaire's disease in humans. D. discoideum is also a host for Legionella and is a suitable model for studying the infection process.[4] Specifically, D. discoideum shares with mammalian host cells a similar cytoskeleton and cellular processes relevant to Legionella infection, including phagocytosis, membrane trafficking, endocytosis, vesicle sorting, and chemotaxis.
Much research has been done to better understand the behavioral responses of D. discoideum. D. discoideum, more specifically the slug form, has an array of behavioral traits. Although it lacks sensory cells and organs it has a keen response to external stimuli, such as temperature and light. Over 100,000 individual amoebae are attracted to one another through chemotaxis. Light is directly related to the cAMP cell-cell signaling in chemotaxis. Within the D. dicoideum the light causes cAMP to be released from the slug tip thus speeding up and directionalizing movement towards the light source. D.discoideum also prefers a slightly acidic pH. When intracellular pH is increased it results in increased locomotion during chemotaxis. The movement of the slug is also increased because the movement of each individual cell has increased.
Lab cultivation
D. discoideum is easy to cultivate in the lab,[3] adding to its appeal as a model organism. It is usually grown in Petri dishes containing nutrient agar and the surface of the dishes are kept moist. The cultures are best grown at 22o-24oC (room temperature). It can also be grown in liquid culture. D. discoideum primarily feeds on E. coli. This substrate is adequate for all stages of the life cycle of this slime mold. When the food supply is diminished, the myxamoebae will aggregate to form pseudoplasmodia. Soon, the dish will be covered with various stages of the life cycle. Check the dish often and make detailed observations of the development. The cells can be harvested at any stage of development and grown quickly.
While cultivating in a lab it is important to take into account D. discoideum's behavioral responses. For example, they have an affinity towards light, higher temperatures, high humidity, low ionic concentrations, and the acidic side of the pH gradient. Experiments are often done to see how manipulations of these parameters hinder, stop, or accelerate development. Variations of these parameters can alter the rate and viability of culture growth. Also the fruiting bodies, being that this is the tallest stage of development, are very responsive to air currents and physical stimuli. It is unknown if there is a stimulus involved with spore release.
Classification and phylogeny
Dictyostelium has maintained more of their ancestral genome diversity than plants and animals, although proteome-based phylogeny confirms that amoebozoa diverged from the animal–fungal lineage after the plant–animal split.[5] The aggregation of individual amoebae into a multicellular fruiting body was an important factor that related the acrasids and dictyostelid cellular slime molds into a larger Class Acrasiomycetes.[6] Subclass, Dictyosteliidae, Order Dictyosteliales is a monophyletic assemblage within the Mycetozoa, a group that includes the protostelid, dictyostelid, and myxogastrid slime molds. Elongation factor-1α (EF-1α) data analyses support Mycetozoa as a monophyletic group even though rRNA trees place it as a polyphyletic group. Further, these data support the idea that the dictyostelid and myxogastrid are more closely related to each other than they are the protostelid. EF-1α analysis also placed the Mycetozoa as the immediate outgroup for the “animal-fungal clade.”[7] Though not strongly supported, there is evidence that Mycetozoa are crown eukaryotes, more closely related to animals and fungi than are the green plants. The multicellularity of the dictyostelid evidences its relationship to animals. Comparing specific amino acid sequences of eight proteins from D. discoideum to those of their homologs in bacteria, yeast, and other eukaryotes indicates that Dictyostelium diverged from the line leading to mammals about the same time as the plant and animal divergence.[8] Therefore, the comparison of these eight proteins shows evidence that mammals are more closely related to Dictyostelium than to yeast. The experiment that was conducted by Loomis and Smith in 1990 suggested that yeast diverged much earlier.
Genome
The D. discoideum genome sequencing project was completed and published in 2005 by an international collaboration of institutes. This was the first free-living protozoan genome to be fully sequenced. D. discoideum consists of a 34Mb haploid genome with a base composition of 77% [A+T] and contains six chromosomes that encode approximately 12,500 proteins.[1] Sequencing of the D. discoideum genome provides a more intricate study of its cellular and developmental biology.
Tandem repeats of trinucleotides are very abundant in this genome; one class of the genome is clustered leading researchers to believe it serves as centromeres. The repeats correspond to repeated sequences of amino acids and are thought to be expanded by nucleotide expansion.[1] Expansion of trinucleotide repeats also occurs in humans, generally leading to many diseases. Learning how D. discoideum cells endure these amino acid repeats may provide insight to allow humans to tolerate them.
Every genome that is sequenced plays an important role in identifying genes that have been gained or lost over time. Due to comparative genomic studies we can compare eukaryotic genomes. A phylogeny based on the proteome showed that the amoebozoa deviated from the animal-fungal lineage after the plant-animal split.[1] The D. discoideum genome is noteworthy because its many encoded proteins are commonly found in fungi, plants, and animals.[1]
Notes
- ^ a b c d e Eichinger, L. "Crawling into a new era--the Dictyostelium genome project". EMBO Journal. 22 (9). European Molecular Biology Organization: 1941–1946.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Eichenger" was defined multiple times with different content (see the help page). - ^ a b c Gilbert, Scott F. (2006). Developmental Biology, Eighth Edition. Sinauer. pp. 36–39. ISBN 087893250X. Cite error: The named reference "Gilbert" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j k l Tyler, Mary S. (2000). Developmental Biology A Guide for Experimental Study, Second Edition. Sinauer. pp. 31–34. ISBN 0878938435.
- ^ Bruhn; et al. (2008). "Dictyostelium, a Tractable Model Host Organism for Legionella". Legionella: Molecular Microbiology. Caister Academic Press. ISBN 978-1-904455-26-4.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ L. Eichinger1*, J. A. Pachebat1,2*, G. Glo¨ ckner3*, M.-A. Rajandream4*, R. Sucgang5*,M. Berriman4, J. Song5, R. Olsen9, K. Szafranski3, Q. Xu6,7, B. Tunggal1, S. Kummerfeld2, M. Madera2, B. A. Konfortov2, F. Rivero1, A. T. Bankier2, R. Lehmann3, N. Hamlin4, R. Davies4, P. Gaudet10, P. Fey10, K. Pilcher10, G. Chen5, D. Saunders4, E. Sodergren6,8, P. Davis4, A. Kerhornou4, X. Nie5, N. Hall4†, C. Anjard9, L. Hemphill5, N. Bason4, P. Farbrother1, B. Desany5, E. Just10, T. Morio11, R. Rost12, C. Churcher4, J. Cooper4, S. Haydock13, N. van Driessche6, A. Cronin4, I. Goodhead4, D. Muzny8, T. Mourier4, A. Pain4,M. Lu5, D. Harper4, R. Lindsay5, H. Hauser4, K. James4,M. Quiles8,M. Madan Babu2, T. Saito14, C. Buchrieser15, A. Wardroper2,16, M. Felder3, M. Thangavelu17, D. Johnson4, A. Knights4, H. Loulseged8, K. Mungall4, K. Oliver4, C. Price4, M. A. Quail4, H. Urushihara11, J. Hernandez8, E. Rabbinowitsch4, D. Steffen8, M. Sanders4, J. Ma5, Y. Kohara18, S. Sharp4, M. Simmonds4, S. Spiegler4, A. Tivey4, S. Sugano19, B. White4, D. Walker4, J. Woodward4, T. Winckler20, Y. Tanaka11, G. Shaulsky6,7, M. Schleicher12, G. Weinstock6,8, A. Rosenthal3, E. C. Cox21, R. L. Chisholm10, R. Gibbs6,8, W. F. Loomis9, M. Platzer3, R. R. Kay2, J. Williams22, P. H. Dear2, A. A. Noegel1, B. Barrell4 & A. Kuspa5,6. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature. 435:34-57.
- ^ Cavender, James C. "Taxonomy, slime molds, and the questions we ask". The Mycological Society of America. 96 (6): 968–979.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Baldauf, Sandra L. "Origin and evolution of the slime molds (Mycetozoa)". Evolution. 94: 12007–12012.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Loomis, W. "Molecular phylogeny of Dictyostelium discoideum by protein sequence comparison". Evolution. 87: 9093–9098.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
References
Tyler, Mary (2000). Developmental Biology A Guide for Experimental Study, Second Edition. Sinauer. pp. 31–34. ISBN 0878938435.
Gilbert, Scott F. (2006). Developmental Biology, Eighth Edition. Sinauer. pp. 36–39. ISBN 087893250X.
