Silver fulminate
Template:Chembox new Silver fulminate (AgCNO) is an explosive ionic compound of silver and the fulminate anion.
Silver fulminate is a primary explosive that has very little practical value due to its extreme sensitivity. The impact of a single water droplet has been known to detonate several milligrams of silver fulminate. Even small amounts of this explosive can cause extensive shrapnel damage, and should be treated with extreme caution.
Silver fulminate was first prepared in 1800 by Edward Charles Howard in his research project to prepare a large variety of fulminates. For two hundred years it has been only useful as a curiosity explosive in toys and tricks.
Preparation
This compound can be prepared by the reaction of concentrated nitric acid with silver metal and ethyl alcohol, under careful control of the reaction conditions, to avoid explosion.[citation needed] Only very tiny amounts of silver fulminate should be prepared at once, as even the weight of the crystals can cause them to self detonate.
Silver fulminate can be prepared unintentionally, when an acidic solution of silver nitrate comes in contact with alcohol. This is a hazard in e.g. chemical silvering of mirrors.
Structure
Silver fulminate occurs in two polymorphic forms, an orthorhombic one and a trigonal one with a rhombohedral lattice[1]. The trigonal polymorph consists of cyclic hexamers, (AgCNO)6[2].
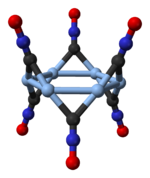 |
hexamer in trigonal silver fulminate |
Uses
Silver fulminate, often in combination with potassium chlorate, is used in trick noise-makers known as "crackers", "snappers", "pop-its", or "bang-snaps", a popular type of novelty firework.
Silver fulminate and "fulminating silver"
Silver fulminate is often confused with silver nitride, silver azide, or fulminating silver[who?]. "Fulminating silver", though always referring to an explosive silver-containing substance, is an ambiguous term. It may be a synonym of silver fulminate. It may also refer to a mixture decomposition product of Tollen's reagent, or an alchemical substance, neither of which may contain the fulminate anion.[who?]
References
- ^ D. Britton and J. D. Dunitz (1965). "The crystal structure of silver fulminate". Acta Cryst. 19 (4): 662–668. doi:10.1107/S0365110X6500405X.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ D. Britton (1991). "A redetermination of the trigonal silver fulminate structure". Acta Cryst. C47 (12): 2646–2647. doi:10.1107/S0108270191008855.
{{cite journal}}: Unknown parameter|month=ignored (help)
Further reading
- K. Singh (1959). "Crystal structure of silver fulminate". Acta Cryst. 12: 1053. doi:10.1107/S0365110X5900295X.
