Formal charge

In chemistry, a formal charge (FC) is a partial charge on an atom in a molecule assigned by assuming that electrons in a chemical bond are shared equally between atoms, regardless of relative electronegativity [1] or in another definition the charge remaining on an atom when all ligands are removed homolytically [2].
The formal charge of any atom in a molecule can be calculated by the following equation: FC = number of valence electrons of the atom in isolation - number of lone pair electrons on this atom in the molecule - half the total number of electrons participating in covalent bonds with this atom in the molecule.
When determining the correct Lewis structure (or predominant resonance structure) for a molecule, the structure is chosen such that the formal charge on each of the atoms is minimized.
Examples:
- carbon in methane: FC = 4 - 0 - 8/2 = 0
- Nitrogen in the nitro group NO2-: FC = 5 - 2 - 6/2 = 0
- double bonded oxygen in NO2-: FC = 6 - 4 - 4/2 = 0
- single bonded oxygen in NO2- FC = 6 - 6 - 2/2 = -1
An alternative method for assigning charge to an atom taking into account electronegativity is by oxidation number. Other related concepts are valence which counts number of electrons that an atom uses in bonding and coordination number, the number of atoms bonded to the atom of interest.
Examples
Ammonium NH4+ is a cationic species. By using the vertical groups of the atoms on the periodic table it is possible to determine that each hydrogen contributes 1 electron, the nitrogen contributes 5 electrons, and the charge of +1 means that 1 electron is absent. The final total is 8 total electrons (1 × 4 + 5 − 1). Drawing the Lewis structure gives an sp3 (4 bonds) hybridized nitrogen atom surrounded by hydrogen. There are no lone pairs of electrons left. Thus, using the definition of formal charge, hydrogen has a formal charge of zero (1-(0 + ½ × 2)) and nitrogen has a formal charge of +1 (5−(0 + ½ × 8)). After adding up all the formal charges throughout the molecule the result is a total formal charge of +1, consistent with the charge of the molecule given in the first place.
Note: The total formal charge in a molecule should be as close to zero as possible, with as few charges on the molecule as possible
- Example: CO2 is a neutral molecule with 16 total valence electrons. There are three different ways to draw the Lewis structure
- Carbon single bonded to both oxygen atoms (carbon = +2, oxygens = -1 each, total formal charge = 0)
- Carbon single bonded to one oxygen and double bonded to another (carbon = +1, oxygendouble = 0, oxygensingle = −1, total formal charge = 0)
- Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge =0)
Even though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all
Alternative method
Although the formula given above is correct, it is often unwieldy and inefficient to use. A much quicker and still accurate method is to do the following:
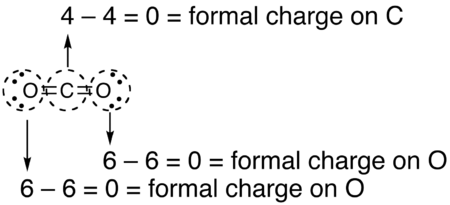
- Draw a circle around the atom for which the formal charge is requested (as with carbon dioxide, below)
- Count up the number of electron in the atom's "circle." Since the circle cuts the covalent bond "in half," each covalent bond counts as one electron instead of two.
- Subtract the number of electrons in the circle from the group number of the element (the roman numeral from the older system of group numbering, NOT the IUPAC 1-18 system) to determine the formal charge. (aka: old group number minus electrons in circle)
- The formal charges computed for the remaining atoms in this Lewis structure of carbon dioxide are shown below.
Again, this method is just as accurate as the one cited above, but is much easier to use. It is important to keep in mind that formal charges are just that-formal, in the sense that this system is a formalism. Atoms in molecules do not have "signs around their necks" indicating their charge. The formal charge system is just a method to keep track of all of the valence electrons that each atom brings with it when the molecule is formed.
Formal Charge vs. Oxidation State
The concept of oxidation states constitutes a competing method to assess the distribution of electrons in molecules. If the formal charges and oxidation states of the atoms in carbon dioxide are compared, the following values are arrived at:
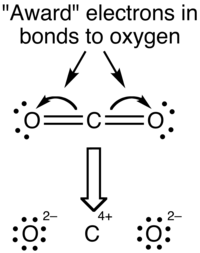
The reason for the difference between these values is that formal charges and oxidation states represent fundamentally different ways of looking at the distribution of electrons amongst the atoms in the molecule. With formal charge, the electrons in each covalent bond are assumed to be split exactly evenly between the two atoms in the bond (hence the dividing by two in the method described above). The formal charge view of the CO2 molecule is essentially shown below:
The covalent (sharing) aspect of the bonding is overemphasized in the use of formal charges, since in reality there is a higher electron density around the oxygen atoms due to their higher electronegativity compared to the carbon atom. This can be most effectively visualized in an electrostatic potential map.
With the oxidation state formalism, the electrons in the bonds are "awarded" to the atom with the greater electronegativity. The oxidation state view of the CO2 molecule is shown below:
Oxidation states overemphasize the ionic nature of the bonding; most chemists agree that the difference in electronegativity between carbon in oxygen is insufficient to regard the bonds as being ionic in nature.
In reality, the distribution of electrons in the molecule lies somewhere between these two extremes. The inadequacy of the simple Lewis structure view of molecules led to the development of the more generally applicable and accurate valence bond theory of Slater, Pauling, et al., and thenceforth the molecular orbital theory developed by Mulliken and Hund.
References
- ^ Lewis Structure Representation of Free Radicals Similar to ClO Hirsch, Warren; Kobrak, Mark. J. Chem. Educ. 2007, 84, 1360. Abstract
- ^ Valence, Oxidation Number, and Formal Charge: Three Related but Fundamentally Different Concepts Parkin, Gerard J. Chem. Educ. 2006, 83, 791. Abstract