Triflic acid

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Trifluoromethanesulfonic acid
| |
| Other names
Triflic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.625 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CF3SO3H | |
| Molar mass | 150.08 g/mol |
| Appearance | Colorless liquid |
| Density | 1.696 g/mL |
| Melting point | −40 °C |
| Boiling point | 162 °C |
| Miscible | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive, eye irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.[1][2]
Properties
Triflic acid is a hygroscopic, colorless liquid at room temperature. It is soluble in polar solvents such as DMF, DMSO, acetonitrile, and dimethyl sulfone. Addition of triflic acid to polar solvents can however be dangerously exothermic.
With an Ka = 8.0 ×1014 (pKa ~ −15) mol/kg, HOTf qualifies as a superacid. Triflic acid owes many of its useful properties to its great thermal and chemical stability. Both the acid and its conjugate base CF3SO−
3, known as triflate, resist oxidation/reduction reactions, whereas many strong acids are oxidizing, e.g. HClO4 and HNO3. Further recommending its use, triflic acid does not sulfonate substrates, which can be a problem with sulfuric acid, fluorosulfuric acid, and chlorosulfonic acid. Below is a prototypical sulfonation, which HOTf does not undergo:
- C6H6 + H2SO4 → C6H5(SO3H) + H2O
Triflic acid fumes in moist air and forms a stable solid monohydrate, CF3SO3H·H2O, melting point 34 °C.
Health Precautions
Trifluouromethanesulfonic acid is one of the strongest acids known. On eye contact it causes severe eye burns and may cause blindness. With contact with skin it causes severe burns with delayed tissue destruction. On inhalation it causes fatal spasms, inflammation and edema.[3]
Syntheses
Trifluoromethanesulfonic acid was first synthesized in 1954 by Haszeldine and Kidd by the following reaction:[4]
Trifluoromethanesulfonic acid is produced industrially by electrochemical fluorination (ECF) of methanesulfonic acid:
- CH3SO3H + 4 HF → CF3SO2F + H2O + 1.5 H2
The resulting CF3SO2F is hydrolyzed, and the resulting triflate salt is preprotonated. Alternatively, trifluoromethanesulfonic acid arises by oxidation of trifluoromethylsulfenyl chloride:[5]
- CF3SCl + 2 Cl2 + 2 H2O → CF3SO2OH + 4 HCl
Triflic acid is purified by distillation from triflic anhydride.[2]
Trifluoromethanesulfonic acid is produced in Europe, Asia, North America, Latin American countries. UK, Russian Federation, Germany, Belgium, China, India, USA, etc form the list of producers. China is among leading manufacturing locations for trifluoromethanesulfonic acid.[6]
Uses
In the laboratory, triflic acid is useful in protonations because the conjugate base of triflic acid is non-nucleophilic. It is also used as an acidic titrant in non-aqueous acid-base titration because it behaves as a strong acid in many solvents (acetonitrile, acetic acid, etc.) where common mineral acids (such as HCl or H2SO4) are only moderately strong.
Salt formation
Trifluoromethanesulfonic acid exothermically reacts with metal carbonates and hydroxides. Illustrative is the synthesis of Cu(OTf)2.[7]
- CuCO3 + 2 CF3SO3H → Cu(O3SCF3)2 + H2O + CO2
Chloride ligands can be converted to the corresponding triflates:
- 3 CF3SO3H + [Co(NH3)5Cl]Cl2 → [Co(NH3)5O3SCF3](O3SCF3)2 + 3 HCl
This conversion is conducted in neat HOTf at 100 °C, followed by precipitation of the salt by the addition of ether.
Organic reactions
Triflic acid reacts with acyl halides to give mixed triflate anhydrides, which are strong acylating agents, e.g. in Friedel-Crafts reactions.
- CH3C(O)Cl + CF3SO3H → CH3C(O)OSO2CF3 + HCl
- CH3C(O)OSO2CF3 + C6H6 → CH3C(O)C6H5 + CF3SO3H
Triflic acid catalyzes the reaction of aromatic compounds with sulfonyl chlorides, probably also via the intermediacy of a mixed anhydride of the sulfonic acid.
Triflic acid promotes other Friedel-Crafts-like reactions including the cracking of alkanes and alkylation of alkenes, which are very important to the petroleum industry. These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum-based fuel.
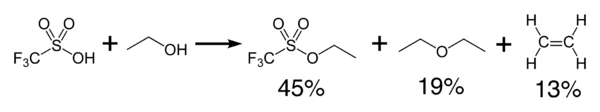
Triflic acid reacts exothermically with alcohols to produce ethers and olefins.
References
- ^ Howells, R. D., McCown, J. D. (1977). "Trifluoromethanesulfonic Acid and Derivatives". Chemical Reviews. 77: 69–92. doi:10.1021/cr60305a005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Subramanian, Lakshminarayanapuram R.; Martãnez, Antonio GarcÃa; Hanack, Michael; Surya Prakash, G. K.; Hu, Jinbo (2006). "Trifluoromethanesulfonic Acid". Encyclopedia of Reagents for Organic Synthesis. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289X.rt246.pub2. ISBN 0471936235.
{{cite encyclopedia}}: soft hyphen character in|first2=at position 14 (help); soft hyphen character in|last2=at position 6 (help) - ^ Catalog of Chemical Suppliers, Buyers, Custom Synthesis Companies And Equipment Manufacturers. "Trifluoromethanesulfonic acid". Retrieved 29 March 2011.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ R. N. Haszeldine and J. M. Kidd (1954). "Perfluoroalkyl derivatives of sulphur. Part I. Trifluoromethanesulphonic acid". J. Chem. Soc.: 4228–4232. doi:10.1039/JR9540004228.
- ^ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, 2007. doi:10.1002/14356007.a11_349
- ^ "Trifluoromethanesulfonic acid (CAS 1493-13-6) is an efficient catalyst in a number of reactions of isomerization, alkylation, and acylation".
- ^ Dixon, N. E.; Lawrance, G. A.; Lay, P. A.; Sargeson, A. M.; Taube, H. (1990). "Trifluoromethanesulfonates and trifluoromethanesulfonato-O complexes". Inorganic Syntheses. Inorganic Syntheses. 28: 70–76. doi:10.1002/9780470132593.ch16. ISBN 9780470132593.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

