Beringian wolf
| Beringian wolf | |
|---|---|

| |
| Beringian wolves diorama at the Yukon Beringia Interpretive Centre | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | |
| Subspecies: | not named
|

The Beringian wolf was an Ice Age gray wolf (Canis lupus) that once inhabited modern day eastern Alaska, the Yukon and northern Wyoming. Some of these wolves survived well into the Holocene but they are now extinct. The Beringian wolf is notable because it was the first gray wolf ecomorph to be identified and comprehensively studied using a range of scientific techniques, which revealed the prey species and feeding behavior of prehistoric wolves. These wolves were found to be morphologically distinct from modern North American wolves and genetically basal to most modern and extinct wolves studied.
Compared with the modern Yukon wolf and other Late Pleistocene gray wolves, the Beringian wolf was similar in size but was more robust with stronger jaws and teeth, a broader palate and larger carnassial teeth relative to its skull size. In comparison with the Beringian wolf the more southerly occurring dire wolf (Canis dirus) was the same size but heavier and possessed a more robust skull and dentition. The unique adaptation of the skull and dentition of the Beringian wolf allowed it to produce relatively large bite forces, grapple with large struggling prey, and therefore to predate and scavenge on Pleistocene megafauna. The Beringian wolf preyed most often on horse and steppe bison, and also on caribou, mammoth, and woodland muskox.
At the close of the Ice Age with the loss of cold and dry conditions and the extinction of much of its prey, the Beringian wolf became extinct. The extinction of its prey has been attributed to the impact of climate change, competition with other species including humans, or a combination of both. Local genetic populations were replaced by others within the same species or by others within the same genus. Of the North American wolves, only the ancestor of the modern North American gray wolf survived. The remains of ancient wolves with similar skull and dentition have been found in western Beringia (north-east Siberia). In 2016, a genetic study showed that some of the wolves now living in remote China and Mongolia are genetically identical to one 28,000 year old eastern Beringian wolf specimen, indicating that these share a common maternal ancestor.
Taxonomy
From the 1930s a representative of the American Museum of Natural History worked with the University of Alaska and the Fairbanks Exploration Company to recover specimens uncovered by hydraulic gold dredging near Fairbanks, Alaska. Between 1932-1953 twenty-eight wolf skulls were recovered from the Ester, Cripple, Engineer, and Little Eldorado creeks located north and west of Fairbanks. The skulls were thought to be 10,000 years old. The geologist and paleontologist Theodore Galusha, who helped amass the Frick collections of fossil mammals at the American Museum of Natural History, worked on the wolf skulls over a number of years and noted that compared with modern wolves they were "short-faced".[1] The paleontologist Stanley John Olsen continued Galusha's work with the short-faced wolf skulls, and in 1985 based on their morphology he classified them as Canis lupus (gray wolf).[2]
Olsen described the short-faced wolf skulls as follows:
The proportions of the skulls of these wolves that vary do so in the rostral area. The area of the skull that is anterior to the infraorbital foramen is noticeably foreshortened and constricted laterally in several of the skulls...Dishing of the rostrum, when viewed laterally, is evident in all of the short-faced skulls identified as Canis lupus from the Fairbanks gold fields. The occipital and supraoccipital crests are noticeably diminished compared to those found in average specimens of C. lupus. The occipital overhang of these crests, a wolf characteristic, is about equal in both groups of C. lupus.[1][2]...Examination of a large series of recent wolf skulls from the Alaskan area did not produce individuals with the same variations as those from the Fairbanks gold fields.[2]
A 2007 genetic, morphology, and stable isotope analyses of seventy-four Beringian wolf specimens from Alaska and the Yukon revealed the genetic relationships, prey species, and feeding behavior of prehistoric wolves. The study supported the Beringian wolf being classified as C. lupus.[3][4] The specimens were not assigned a sub-species classification but they were referred to as "eastern Beringian wolves".[5][a] Beringia was an area of land that once spanned the Chukchi Sea and the Bering Sea joining Eurasia to North America, when eastern Beringia included what is today Alaska and the Yukon.
Lineage
Basal wolf
DNA sequences can be mapped to reveal a phylogenetic tree that represents evolutionary relationships, with each branch point representing the proposed divergence of two lineages from a common ancestor. On this tree the term basal is used to describe a lineage that forms a branch diverging nearest to the common ancestor.[6] Studies have found the Beringian wolf to be basal to all other gray wolves except for the modern Indian gray wolf and Himalayan wolf,[4] and the extinct Belgian clade of Pleistocene wolves.[7][8]
Two types of gray wolf
A haplotype is a group of genes found in an organism that are inherited together from one of their parents.[9][10] A haplogroup is a group of similar haplotypes that share a single mutation inherited from their common ancestor.[11][12] Mitochondrial DNA (mDNA) passes along the maternal line and can date back thousands of years.[11] A 2005 study compared mitochondrial DNA sequences of modern wolves with those from thirty-four specimens dated between 1856-1915. The historic population was found to possess twice the genetic diversity of modern wolves.[13][14] A 2007 study compared mDNA sequences of modern wolves with those from Beringian wolves. The twenty Beringian wolves yielded sixteen haplotypes that were not found in modern wolves, compared to seven haplotypes found in thirty-two modern Alaskan and Yukon wolves. This finding indicates that Beringian wolves were genetically distinct from modern wolves, [4][13][15] that they possessed greater genetic diversity, and there was a larger wolf population than today.[4]
| Phylogenetic tree for wolves | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| Simplified mDNA phylogeny for modern wolves and extinct Beringian wolves[4][16] |
A 2010 study compared mDNA sequences of modern wolves with those from 24 ancient wolf specimens from western Europe dated between 44,000–1,200 years before present (YBP). The study found that the sequences could be allocated into two haplogroups.[4][13][17] Haplogroups 1 and 2 could be found among wolves across Eurasia but only haplogroup 1 could be found in North America. The ancient wolf samples from western Europe differed from modern wolves by 1 to 10 mutations, and all belonged to haplogroup 2 indicating its predominance in this region for over 40,000 years, both before and after the Last Glacial Maximum. A comparison of current and past frequencies indicated that in Europe haplogroup 2 became outnumbered by haplogroup 1 over the past several thousand years but in North America haplogroup 2 — including the Beringian wolf — became extinct and was replaced by haplogroup 1 after the Last Glacial Maximum.[17][16]
A scenario consistent with the phylogenetic, ice sheet size and sea-level depth data is that during the Late Pleistocene the sea levels were at their lowest. A single wave of wolf colonization into North America commenced with the opening of the Bering land bridge 70,000 YBP and ending with the closing of the Yukon corridor that ran between the division between the Laurentide Ice Sheet and the Cordilleran Ice Sheet 23,000 YBP during the Late Glacial Maximum. As wolves had been in the fossil record of North America but modern wolves could trace their genetic ancestry back only 80,000 years,[7][8] the wolf haplotypes that were already in North America were replaced by these invaders, either through competitive displacement or through admixture. The replacement in North America of a basal population of wolves by a more recent one supports the findings of earlier studies.[4][8][17][16] There possibly existed a panmictic (random mating) wolf population with gene flow spanning Eurasia and North America until the closing of the ice sheets.[8][16][18] Once the sheets closed, the southern wolves were isolated and north of the sheets only the Beringian wolf existed. The land bridge became inundated by the sea 10,000 YBP and the ice sheets receded 12,000—6,000 YBP.[8] The Beringian wolf became extinct and the southern wolves expanded through the shrinking ice sheets to recolonize the northern part of North America.[8][18] All North American wolves are descended from those that were once isolated south of the ice sheets. However, much of their diversity was later lost during the twentieth century.[8][14]
Description

The Beringian wolf was similar in size to the modern Yukon wolf (C. l. pambasileus).[4] The largest northern wolves today have a shoulder height not exceeding 97 cm (38 in) and a body length not exceeding 180 cm (71 in).[19]: 1 The average weight of the Yukon wolf is 43 kg (95 lb) for males and 37 kg (82 lb) for females. Individual weights for Yukon wolves can vary from 21 kg (46 lb) to 55 kg (121 lb),[20] with one Yukon wolf weighing 79.4 kg (175 lb).[19]: 1 The Beringian wolves were similar in size to the Late Pleistocene wolves whose remains have been found in the La Brea Tar Pits at Los Angeles, California.[4] These wolves, referred to as Rancho La Brea wolves, were not physically different from modern gray wolves, with the only differences being a broader femur bone and a longer tibial tuberosity — the insertion for the quadriceps and hamstring muscles — which provides evidence that they possessed comparatively more powerful leg muscles for a fast take-off before a chase.[21] The Beringian wolf was more robust with stronger jaws and teeth than Rancho La Brea or modern wolves.[4][13]
During the Late Pleistocene, the more southerly occurring dire wolf (Canis dirus) had the same shape and proportions as the Yukon wolf,[22][23] however C. dirus guildayi is estimated to have weighed on average 60 kg (130 lb), and C. dirus dirus weighed on average 68 kg (150 lb) with some specimens being larger.[24] The dire wolf was heavier than the Beringian wolf and possessed a more robust skull and dentition.[4]
Adaptation
Adaptation is the evolutionary process in which an organism becomes better able to live in its habitat or habitats.[25] Ecological factors such as habitat type, climate, prey specialization, and predatory competition have been shown to greatly influence gray wolf craniodental plasticity, which is an adaptation of the cranium and teeth due to the influences of the environment.[26][27][28] In the Late Pleistocene, the variations between local environments would have encouraged a range of wolf ecotypes that were genetically, morphologically and ecologically distinct from each another.[26] The term ecomorph is used to describe a habitat specialist.[29] The Beringian wolf ecomorph shows evolutionary craniodental plasticity not seen in past nor present North American gray wolves.[4]
Paleoecology

The last glacial period, commonly referred to as the "Ice Age", spanned 125,000[30]–14,500 YBP[31] and was the most recent glacial period within the current ice age, which occurred during the last years of the Pleistocene era.[30] The Ice Age reached its peak during the Last Glacial Maximum, when ice sheets began advancing from 33,000 YBP and reached their maximum limits 26,500 YBP. Deglaciation commenced in the Northern Hemisphere approximately 19,000 YBP and in Antarctica approximately 14,500 years YBP, which is consistent with evidence that glacial meltwater was the primary source for an abrupt rise in sea level 14,500 YBP[31] and the Bering land bridge was finally inundated around 11,000 YBP.[32] The fossil evidence from many continents points to the extinction of large animals, termed Pleistocene megafauna, near the end of the last glaciation.[33]
During the Ice Age a vast, cold and dry Mammoth steppe stretched from the arctic islands southwards to China, and from Spain eastwards across Eurasia and over the Bering land bridge into Alaska and the Yukon where it was blocked by the Wisconsin glaciation. The land bridge existed because sea-levels were lower due to more of the planet's water being locked up in glaciers compared with today. Therefore, the flora and fauna of Beringia were more related to those of Eurasia rather than those of North America.[34][35] In eastern Beringia from 35,000 YBP the northern arctic areas experienced temperatures 1.5°C (2.7°F) warmer than today but the southern sub-Arctic regions were 2°C (3.5°F) cooler. During the Last Glacial Maximum, in 22,000 YBP the average summer temperature was 3-5°C (5.4-9°F) cooler than today with variations of 2.9°C (5.2°F) cooler on the Seward Peninsula to 7.5°C (13.5°F) cooler in the Yukon.[36]
Beringia received more moisture and intermittent maritime cloud cover from the north Pacific Ocean than the rest of the Mammoth steppe, including the dry environments on either side of it. Moisture occurred along a north-south gradient with the south receiving the most cloud cover and moisture due to the air-flow from the North Pacific.[35] This moisture supported a shrub-tundra habitat that provided a ecological refugium for plants and animals.[34][35] Eastern Beringia's vegetation included isolated refugia of larch and spruce forests with birch and alder trees.[37][38][39][40] This environment supported large herbivores that were prey for Beringian wolves and their competitors. Steppe bison (Bison priscus), Yukon horse (Equus lambei), mammoth (Mammuthus primigenius) and Wild yak (Bos mutus) consumed grasses, sedges, and herbaceous plants. Caribou (Rangifer tarandus) and woodland muskox (Symbos cavifrons) consumed tundra plants, including lichen, fungi, and mosses.[41]
Prey

Isotope analysis can be used to identify some chemical elements, allowing researchers to make inferences about the diet of the species being studied. Two isotope analyses of bone collagen extracted from the remains of Late Pleistocene wolves found in Beringia and Belgium indicate that they both preyed mainly on Pleistocene megafauna,[4][16][42] which became rare at the beginning of the Holocene 12,000 years ago.[16][43] The Beringian wolf preyed most often on horse and steppe bison.[4][15] In the period leading up to the Last Glacial Maximum (50,000 YBP-23,000 YBP) they also ate woodland muskox, and after this time they also ate mammoth. The analysis supports the conclusion that these wolves were capable of killing and dismembering large prey.[4]
In another stable isotope analysis, half of the Beringian wolves were found to be muskox and caribou specialists, and the other half were either horse and bison specialists or generalists. Two wolves from the full-glacial period (23,000–18,000 YBP) were found to be mammoth specialists but it is not clear if this was due to scavenging or predation. The analysis of other carnivore fossils from the Fairbanks region of Alaska found that mammoth was rare in the diets of the other Beringian carnivores.[41]
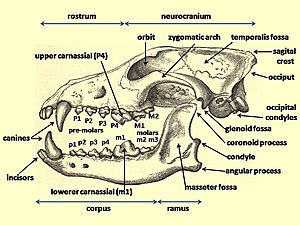
Dentition
A study of Canis dentition shows that in comparison with the modern gray wolf and the Pleistocene La Brea wolf, the Beringian wolf possessed large carnassial teeth and a short, broad palate relative to the size of its skull. The row length of the Beringian wolf's premolars was longer, the P4 premolar (the upper carnassial) longer and wider, and the M1, M2, and m1 (the lower carnassial) molars longer than those found in the other two wolves. The Beringian wolf's short, broad rostrum increased the force of a bite made with the canine teeth while strengthening the skull against the stresses caused by struggling prey. Today, the relatively deep jaws similar to those of the Beringian wolf can be found in the bone-cracking spotted hyena and in those canids that are adapted for taking large prey.[4] Beringian wolves possessed a craniodental morphology that was more specialized than modern gray wolves and Rancho La Brea wolves for capturing, dismembering, and consuming the bones of very large megaherbivores,[4][15] and they had evolved this way due to the presence of megafauna.[44] Their stronger jaws and teeth indicate a hypercarnivorous lifestyle[4][13]
| Tooth variable | modern North America | Rancho La Brea | Eastern Beringia |
|---|---|---|---|
| premolar row length | 63.4 | 63.6 | 69.3 |
| palate width | 64.9 | 67.6 | 76.6 |
| P4 length | 25.1 | 26.3 | 26.7 |
| P4 width | 10.1 | 10.6 | 11.4 |
| M1 length | 16.4 | 16.5 | 16.6 |
| M2 length | 8.7 | 8.9 | 9.2 |
| m1 length | 28.2 | 28.9 | 29.6 |
| m1 trigonid length | 19.6 | 21.9 | 20.9 |
| m1 width | 10.7 | 11.3 | 11.1 |
Tooth breakage

Tooth breakage is related to a carnivore's behaviour.[45] The mandibles of canids are buttressed behind the carnassial teeth to crack bones with their post-carnassial teeth (molars M2 and M3). A study found that the modern gray wolf possesses greater buttressing when compared to all other extant canids and the extinct dire wolf. This indicates that the gray wolf is better adapted for cracking bone than other canids.[46] In comparison to extant North American gray wolves, Beringian wolves included many more individuals with moderately to heavily worn teeth and with a significantly greater number of broken teeth. The frequencies of fracture ranged from a minimum of 2% found in the Northern Rocky Mountain wolf (Canis lupus irremotus) up to a maximum of 11% found in Beringian wolves. The distribution of fractures across the tooth row also differs, with Beringian wolves having much higher frequencies of fracture for incisors, carnassials, and molars. A similar pattern was observed in spotted hyenas, suggesting that increased incisor and carnassial fracture reflects habitual bone consumption because bones are gnawed with the incisors and then cracked with the carnassials and molars.[4] The risk of tooth fracture is also higher when taking and consuming large prey.[47][48]
Competitors
The Beringian carnivores included the Beringian wolf (Canis lupus), Beringian cave lion (Panthera leo spelaea), scimitar-toothed cat (Homotherium serum), short-faced bear (Arctodus simus), and the omnivorous brown bear (Ursus arctos).[41] Beringian wolves would have faced competition for the carcasses of large herbivores from the formidably-large short-faced bear, a scavenger.[49] Compared to modern wolves, the high frequency of tooth fracture in Beringian wolves indicates higher carcass consumption due to higher carnivore density and increased competition.[4] Additionally, humans had reached the Bluefish Caves in the Yukon Territory by 24,000 YBP,[50] introducing a new competitor for large game.
A 1993 study proposed that the higher frequency of tooth breakage among Pleistocene carnivores compared with living carnivores was not the result of hunting larger game, something that might be assumed from the larger size of the former. When there is low prey availability, the competition between carnivores increases, causing them to eat faster and thus consume more bone, leading to tooth breakage.[45][51][52]
Range
Beringian wolves have been found in Alaska and as far eastward as the Yukon in Canada.[5] They are morphologically and genetically comparable to Late Pleistocene European wolves[53] and two studies found that some share a common mDNA haplotype.[16][4]
One study found that ancient wolves across Eurasia had identical mDNA sequence as six Beringian wolves (indicating a common maternal ancestor). These wolves included a wolf from the Nerubajskoe-4 Paleolithic site, near Odessa, Ukraine dated 30,000 YBP, a wolf from the Zaskalnaya-9 Paleolithic site, Zaskalnaya on the Crimean Peninsula dated 28,000 YBP, and the "Altai dog" from the Altai Mountains of Central Asia dated 33,000 YBP. Another wolf from the Vypustek cave, Czech Republic dated 44,000 YBP had an identical mDNA sequence to two Beringian wolves (indicating another common maternal ancestor).[4]

The Beringian wolves are phylogenetically associated with a distinct group of four modern European mDNA haplotypes, which indicates that both ancient and extant North American wolves originated in Eurasia.[4] Of these four modern haplotypes, one was only found in the Italian wolf and one only found in Rumania.[54] These four haplotypes fall with all of the Beringian wolf haplotypes under mDNA haplogroup 2.[16] Ancient specimens of wolves with similar skull and dentition have been found in western Beringia (northeast Siberia), the Taimyr Peninsula, the Ukraine, and in Germany where the European specimens are classified as Canis lupus spelaeus—the cave wolf.[55] The Beringian wolves, and perhaps wolves across the Mammoth steppe, were adapted to preying on now-extinct species through their unique skull and tooth morphology.[27]
Specimens that have been identified by their skull morphology to be Beringian wolves have been found in the Natural Trap Cave at the base of the Bighorn Mountains in Wyoming, United States. These were radiocarbon dated between 25,800-14,300 YBP and this location is directly south of what would at that time have been the division between the Laurentide Ice Sheet and the Cordilleran Ice Sheet. This suggests that a temporary channel existed between the glaciers from 25,800 YBP[5] until the advance of the ice sheets 16,000-13,000 YBP.[5][56] The migration of the Beringian wolf is assumed to have been the result of pursuing prey species, as this cave also contained specimens of steppe bison that had migrated from Beringia and would have been prey for wolves,[5][57] and muskox that is known to be an important prey species of the Beringian wolf.[5][41] Dire wolves were absent north of 42°N latitude in the Late Pleistocene, therefore this region would have been available for Beringian wolves to expand southwards. There is no evidence of expansion beyond this region.[5] Another study of bison through this area indicated that this corridor was closed between 23,000 YBP until 13,400 YBP,[58] and if this is correct then it suggests that the wolves were able to migrate south between 23,800-23,000 YBP but were then unable to return north due to this corridor being closed.
Extinction
Extinction is the result of the elimination of the geographic range of a species with a reduction of its population size down to zero. The factors that affect biogeographic range and population size include competition, predator/prey interactions, variables of the physical environment, and chance events.[59]
Phenotype is extinct

The mammoth steppe lasted for 100,000 years without change until it came to an end around 12,000 years ago.[35] The American megafaunal extinction event occurred 12,700 YBP when 90 genera of mammals weighing over 44 kilograms (97 lb) became extinct.[60][51] The extinction of the large carnivores and scavengers is thought to have been caused by the extinction of the megaherbivore prey upon which they depended.[61][62] The cause of the extinction of these megafauna is debated[48] but has been attributed to the impact of climate change, competition with other species including humans, or a combination of both.[48][63] Ancient DNA and radiocarbon data indicates that local genetic populations were replaced by others within the same species or by others within the same genus.[64]

Postglacial environmental change throughout eastern Beringia brought about wholesale changes in vegetation, the regional extinction of much of the megafauna, and the entrance of Homo sapiens.[36] The large Late Pleistocene carnivores that were more carnivorous than their competitors faced greater vulnerability to extinction. The Beringian cave lion, saber-toothed cat, and short-faced bear went extinct at the same time as their large megafaunal prey. The omnivorous coyote, American black bear, brown bear, puma and bob-cat survived. Both the Beringian wolf and the dire wolf went extinct in North America, leaving only the less carnivorous and more gracile form of the wolf to thrive.[4] One extinction theory holds that the Beringian wolf was outcompeted and replaced by the ancestor of the modern gray wolf.[5]
The radio carbon dating of the skeletal remains from 56 Beringian wolves showed a continuous population from over 50,800 YBP[65] until 12,500 YBP, followed by one wolf dated at 7,600 YBP. This indicates that their population was in decline after 12,500 YBP,[4] although megafauna was still available in this region until 10,500 YBP.[66] The timing of this latter specimen is supported by the recovery of mammoth and horse DNA from sediments dated 10,500 YBP–7,600 YBP from the interior of Alaska.[66] The timing for the extinction of horses in North America and the minimum population size for North American bison coincide with the extinction of an entire wolf haplogroup in North America, indicating that the disappearance of their prey caused the extinction of this wolf ecomorph.[4][13][15][43] This resulted in a significant loss of phenotypic and genetic diversity within the species.[4]
Haplotype is not extinct
There are parts of Central Eurasia where the environment is considered to have been stable for the past 40,000 years.[67] In 2016 a study compared mDNA sequences of ancient wolf specimens with those from modern wolves, including specimens from the remote regions of North America, Russia, and China. One ancient haplotype that had once existed in both Alaska (Eastern Beringia 28,000 YBP) and Russia (Medvezya "Bear" Cave, Pechora area, Northern Urals 18,000 YBP) was similar to that of modern wolves found living in Mongolia and China (indicating a common maternal ancestor). The study found that genetic diversity of past wolves was lost at the beginning of the Holocene in Alaska, Siberia, and Europe and there is limited overlap with modern wolves. The study did not support two wolf haplogroups that had been proposed by earlier studies. For the ancient wolves of North America, instead of an extinction/replacement model indicated by other studies this study found substantial evidence of a population bottleneck (reduction) in which the ancient wolf diversity was almost lost at the beginning of the Holocene. In Eurasia, the loss of many ancient lineages cannot be simply explained and appears to have been slow across time with the reasons unclear.[65]
Notes
- ^ This wolf could be associated with the European cave wolf Canis lupus spelaeus.
References
- ^ a b Olsen, Stanley J. (2001). "II.G.8-Domestication:Dogs". In Kiple, Kenneth F.; Ornelas, Kriemhild Coneè (eds.). The Cambridge World History of Food. Vol. 1. Cambridge University Press. pp. 513–514. ISBN 0-521-40214X.
- ^ a b c Stanley J. Olsen (1985). Origins of the Domestic Dog: The Fossil Record – Chapter 2. University of Arizona Press. p. 22.
- ^ Smith, Alison J. (2012). "12 Evidence of Environmental Change from Terrestrial and Freshwater Paleoecology". In John A Matthews (ed.). The SAGE Handbook of Environmental Change. Vol. 1. SAGE Los Angeles. p. 272. ISBN 9780857023605.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab Leonard, Jennifer A.; Vilà, Carles; Fox-Dobbs, Kena; Koch, Paul L.; Wayne, Robert K.; Van Valkenburgh, Blaire (2007). "Megafaunal Extinctions and the Disappearance of a Specialized Wolf Ecomorph" (PDF). Current Biology. 17 (13): 1146. doi:10.1016/j.cub.2007.05.072. PMID 17583509.
- ^ a b c d e f g h i Meachen, Julie A.; Brannick, Alexandria L.; Fry, Trent J. (2016). "Extinct Beringian wolf morphotype found in the continental U.S. Has implications for wolf migration and evolution". Ecology and Evolution. 6 (10): 3430–8. doi:10.1002/ece3.2141. PMC 4870223. PMID 27252837.
- ^ Reece, Jane B.; Meyers, Noel; Urry, Lisa A.; Cain, Michael L.; Wasserman, Steven A.; Minorsky, Peter V.; Jackson, Robert B.; Cooke, Bernard N. (2015). "26-Phylogeny and the tree of life". Campbell Biology Australian and New Zealand version (10 ed.). Pierson Australia. pp. 561–562. ISBN 9781486007042.
- ^ a b Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V. J.; Sawyer, S. K.; Greenfield, D. L.; Germonpre, M. B.; Sablin, M. V.; Lopez-Giraldez, F.; Domingo-Roura, X.; Napierala, H.; Uerpmann, H.-P.; Loponte, D. M.; Acosta, A. A.; Giemsch, L.; Schmitz, R. W.; Worthington, B.; Buikstra, J. E.; Druzhkova, A.; Graphodatsky, A. S.; Ovodov, N. D.; Wahlberg, N.; Freedman, A. H.; Schweizer, R. M.; Koepfli, K.- P.; Leonard, J. A.; Meyer, M.; Krause, J.; Paabo, S.; et al. (2013). "Complete Mitochondrial Genomes of Ancient Canids Suggest a European Origin of Domestic Dogs". Science. 342 (6160): 871. Bibcode:2013Sci...342..871T. doi:10.1126/science.1243650. PMID 24233726.
- ^ a b c d e f g Koblmüller, Stephan; Vilà, Carles; Lorente-Galdos, Belen; Dabad, Marc; Ramirez, Oscar; Marques-Bonet, Tomas; Wayne, Robert K.; Leonard, Jennifer A. (2016). "Whole mitochondrial genomes illuminate ancient intercontinental dispersals of grey wolves (Canis lupus)". Journal of Biogeography. 43 (9): 1728. doi:10.1111/jbi.12765.
- ^ Cox, C. B.; Moore, Peter D.; Ladle, Richard (2016). Biogeography: An Ecological and Evolutionary Approach. Wiley-Blackwell. p. 106. ISBN 978-1-118-96858-1.
- ^ Editorial Board (2012). Concise Dictionary of Science. New Delhi: V&S Publishers. p. 137. ISBN 978-93-81588-64-2.
- ^ a b Arora, Devender; Singh, Ajeet; Sharma, Vikrant; Bhaduria, Harvendra Singh; Patel, Ram Bahadur (2015). "Hgs Db: Haplogroups Database to understand migration and molecular risk assessment". Bioinformation. 11 (6): 272–5. doi:10.6026/97320630011272. PMC 4512000. PMID 26229286.
- ^ "Genetics Glossary". International Society of Genetic Genealogy. 2015. Retrieved 22 July 2016.
- ^ a b c d e f Miklosi, Adam (2015). Dog Behaviour, Evolution, and Cognition. Oxford Biology (2 ed.). Oxford University Press. pp. 106–107. ISBN 978-0199545667.
- ^ a b Leonard, Jennifer A.; Vilà, Carles; Wayne, Robert K. (2005). "Legacy lost: Genetic variability and population size of extirpated US grey wolves (Canis lupus)". Molecular Ecology. 14 (1): 9–17. doi:10.1111/j.1365-294X.2004.02389.x. PMID 15643947.
- ^ a b c d Sam Turvey (2009). Holocene Extinctions. Oxford University Press. p. 257. ISBN 978-0-19-953509-5.
- ^ a b c d e f g h Pilot, Małgorzata; Branicki, Wojciech; Jędrzejewski, Włodzimierz; Goszczyński, Jacek; Jędrzejewska, Bogumiła; Dykyy, Ihor; Shkvyrya, Maryna; Tsingarska, Elena (2010). "Phylogeographic history of grey wolves in Europe". BMC Evolutionary Biology. 10: 104. doi:10.1186/1471-2148-10-104. PMC 2873414. PMID 20409299.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Randi, Ettore (2011). "Genetics and conservation of wolves Canis lupus in Europe". Mammal Review. 41 (2): 99–111. doi:10.1111/j.1365-2907.2010.00176.x.
- ^ a b Hofreiter, Michael (2007). "Pleistocene Extinctions: Haunting the Survivors". Current Biology. 17 (15): 609–11. doi:10.1016/j.cub.2007.06.031. PMID 17686436.
- ^ a b Mech, L. David (1966). The Wolves of Isle Royale. Fauna Series 7. Fauna of the National Parks of the United States. ISBN 1-4102-0249-6. Retrieved 1 May 2017.
- ^ "Gray wolf (in the Yukon)" (PDF). Environment Yukon. Government of Canada. 2017. Retrieved 18 April 2017.
- ^ Meachen, J. A.; Samuels, J. X. (2012). "Evolution in coyotes (Canis latrans) in response to the megafaunal extinctions". Proceedings of the National Academy of Sciences. 109 (11): 4191–6. doi:10.1073/pnas.1113788109. PMC 3306717. PMID 22371581.
- ^ Tedford, Richard H.; Wang, Xiaoming; Taylor, Beryl E. (2009). "Phylogenetic Systematics of the North American Fossil Caninae (Carnivora: Canidae)". Bulletin of the American Museum of Natural History. 325: 1–218. doi:10.1206/574.1.
- ^ Merriam, J. C. (1912). "The fauna of Rancho La Brea, Part II. Canidae". Memoirs of the University of California. 1: 217–273.
- ^ Anyonge, William; Roman, Chris (2006). "New body mass estimates for Canis dirus, the extinct Pleistocene dire wolf". Journal of Vertebrate Paleontology. 26: 209–212. doi:10.1671/0272-4634(2006)26[209:NBMEFC]2.0.CO;2.
- ^ Dobzhansky, Theodosius (1968). "On Some Fundamental Concepts of Darwinian Biology". In Dobzhansky, Theodosius; Hecht, Max K.; Steere, William C. (eds.). Evolutionary Biology. Vol. 2. New York: Appleton-Century-Crofts. pp. 1–34. doi:10.1007/978-1-4684-8094-8_1. OCLC 24875357.
- ^ a b Perri, Angela (2016). "A wolf in dog's clothing: Initial dog domestication and Pleistocene wolf variation". Journal of Archaeological Science. 68: 1–4. doi:10.1016/j.jas.2016.02.003.
- ^ a b Leonard, Jennifer (2014). "Ecology drives evolution in grey wolves" (PDF). 16. Evolution Ecology Research: 461–473.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Flower, Lucy O.H.; Schreve, Danielle C. (2014). "An investigation of palaeodietary variability in European Pleistocene canids". Quaternary Science Reviews. 96: 188–203. Bibcode:2014QSRv...96..188F. doi:10.1016/j.quascirev.2014.04.015.
- ^ Losos, Jonathan B.; Parent, Christina E. (2010). "The Speciation-Area Relationship". In Losos, Jonathan B.; Ricklefs, Robert E. (eds.). The Theory of Island Biogeography Revisited. Princeton University Press. p. 425. ISBN 9780691136523.
- ^ a b Intergovernmental Panel on Climate Change (UN) (2007). "IPCC Fourth Assessment Report: Climate Change 2007 – Palaeoclimatic Perspective". The Nobel Foundation.
- ^ a b Clark, P. U.; Dyke, A. S.; Shakun, J. D.; Carlson, A. E.; Clark, J.; Wohlfarth, B.; Mitrovica, J. X.; Hostetler, S. W.; McCabe, A. M. (2009). "The Last Glacial Maximum". Science. 325 (5941): 710–4. doi:10.1126/science.1172873. PMID 19661421.
- ^ Jakobsson, Martin; Pearce, Christof; Cronin, Thomas M.; Backman, Jan; Anderson, Leif G.; Barrientos, Natalia; Björk, Göran; Coxall, Helen; De Boer, Agatha; Mayer, Larry A.; Mörth, Carl-Magnus; Nilsson, Johan; Rattray, Jayne E.; Stranne, Christian; Semilietov, Igor; o&Amp;apos;regan, Matt (2017). "Post-glacial flooding of the Beringia Land Bridge dated to 11,000 cal yrs BP based on new geophysical and sediment records". Climate of the Past Discussions: 1. doi:10.5194/cp-2017-11.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Elias, S.A.; Schreve, D. (2016). Late Pleistocene Megafaunal Extinctions (PDF). pp. 3202–3217. doi:10.1016/B978-0-12-409548-9.10283-0. ISBN 978-0-12-409548-9.
{{cite book}}:|journal=ignored (help) - ^ a b Elias, S; Crocker, B (2008). "The Bering Land Bridge: A moisture barrier to the dispersal of steppe–tundra biota?". Quaternary Science Reviews. 27 (27–28): 2473. doi:10.1016/j.quascirev.2008.09.011.
- ^ a b c d Dale Guthrie, R (2001). "Origin and causes of the mammoth steppe: A story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia". Quaternary Science Reviews. 20: 549. doi:10.1016/S0277-3791(00)00099-8.
- ^ a b Elias, S.A.; Brigham-Grette, J. (2007). "GLACIATIONS Late Pleistocene Events in Beringia". Encyclopedia of Quaternary Science (PDF). p. 1057. doi:10.1016/B0-44-452747-8/00132-0. ISBN 9780444527479. Retrieved 2 May 2017.
- ^ Hoffecker, JF; Elias, SA (2007). Human ecology of Beringia. Columbia University Press, New York, NY. p. 57. ISBN 9780231130608.
- ^ Brigham-Grette, J; Lozhkin, AV; Anderson, PM; Glushkova, OY (2004). Madsen, DB (ed.). Paleoenvironmental conditions in Western Beringia before and during the Last Glacial Maximim. Published in: Entering America, northeast Asia and Beringia before the last glacial maximum. University of Utah Press, Salt Lake City, Utah. pp. 29–61.
- ^ Sher, A.V.; Kuzmina, S.A.; Kuznetsova, T.V.; Sulerzhitsky, L.D. (2005). "New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals". Quaternary Science Reviews. 24 (5–6): 533. doi:10.1016/j.quascirev.2004.09.007.
- ^ Anderson, Patricia M.; v. Lozhkin, Anatoly (2001). "The Stage 3 interstadial complex (Karginskii/middle Wisconsinan interval) of Beringia: Variations in paleoenvironments and implications for paleoclimatic interpretations". Quaternary Science Reviews. 20: 93–125. doi:10.1016/S0277-3791(00)00129-3.
- ^ a b c d Fox-Dobbs, Kena; Leonard, Jennifer A.; Koch, Paul L. (2008). "Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records". Palaeogeography, Palaeoclimatology, Palaeoecology. 261: 30. doi:10.1016/j.palaeo.2007.12.011.
- ^ Germonpré, Mietje; Sablin, Mikhail V.; Stevens, Rhiannon E.; Hedges, Robert E.M.; Hofreiter, Michael; Stiller, Mathias; Després, Viviane R. (2009). "Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes". Journal of Archaeological Science. 36 (2): 473. doi:10.1016/j.jas.2008.09.033.
- ^ a b Hofreiter, Michael; Barnes, Ian (2010). "Diversity lost: Are all Holarctic large mammal species just relict populations?". BMC Biology. 8: 46. doi:10.1186/1741-7007-8-46. PMC 2858106. PMID 20409351.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stewart, J. R. (2009). "The evolutionary consequence of the individualistic response to climate change". Journal of Evolutionary Biology. 22 (12): 2363–75. doi:10.1111/j.1420-9101.2009.01859.x. PMID 19888939.
- ^ a b Van Valkenburgh, Blaire; Hertel, Fritz (1993). "Tough Times at La Brea: Tooth Breakage in Large Carnivores of the Late Pleistocene" (PDF). Science, New Series. 261 (5120). American Association for the Advancement of Science: 456–459. Bibcode:1993Sci...261..456V. doi:10.1126/science.261.5120.456. PMID 17770024.
- ^ Therrien, François (2005). "Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators". Journal of Zoology. 267 (3): 249. doi:10.1017/S0952836905007430.
- ^ Van Valkenburgh, B (1988). "Incidence of tooth breakage among large predatory mammals". Am. Nat. 131 (2): 291–302. doi:10.1086/284790.
- ^ a b c DeSantis, L.R.G.; Schubert, B.W.; Schmitt-Linville, E.; Ungar, P.; Donohue, S.; Haupt, R.J. (September 15, 2015). John M. Harris (ed.). "Dental microwear textures of carnivorans from the La Brea Tar Pits, California and potential extinction implications". Science Series 42. Contributions in Science (A special volume entitled La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas in commemoration of the 100th anniversary of the Natural History Museum of Los Angeles County's excavations at Rancho La Brea). Natural History Museum of Los Angeles County: 37–52.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Crockford, Susan J.; Kuzmin, Yaroslav V. (2012). "Comments on Germonpré et al., Journal of Archaeological Science 36, 2009 "Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes", and Germonpré, Lázkičková-Galetová, and Sablin, Journal of Archaeological Science 39, 2012 "Palaeolithic dog skulls at the Gravettian Předmostí site, the Czech Republic"". Journal of Archaeological Science. 39 (8): 2797. doi:10.1016/j.jas.2012.04.033.
- ^ Bourgeon, Lauriane; Burke, Ariane; Higham, Thomas (2017). "Earliest Human Presence in North America Dated to the Last Glacial Maximum: New Radiocarbon Dates from Bluefish Caves, Canada". PLoS ONE. 12 (1): e0169486. doi:10.1371/journal.pone.0169486. PMC 5218561. PMID 28060931.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b O'Keefe, F.Robin; Binder, Wendy J.; Frost, Stephen R.; Sadlier, Rudyard W.; Van Valkenburgh, Blaire (2014). "Cranial morphometrics of the dire wolf, Canis dirus, at Rancho La Brea: temporal variability and its links to nutrient stress and climate". Palaeontologia Electronica. 17 (1): 1–24.
- ^ Van Valkenburgh, Blaire (2008). "Costs of carnivory: Tooth fracture in Pleistocene and Recent carnivorans". Biological Journal of the Linnean Society. 96: 68–81. doi:10.1111/j.1095-8312.2008.01108.x.
- ^ Germonpré, Mietje; Sablin, Mikhail V.; Després, Viviane; Hofreiter, Michael; Lázničková-Galetová, Martina; Stevens, Rhiannon E.; Stiller, Mathias (2013). "Palaeolithic dogs and the early domestication of the wolf: A reply to the comments of Crockford and Kuzmin (2012)". Journal of Archaeological Science. 40: 786–792. doi:10.1016/j.jas.2012.06.016.
- ^ Vila, C; Amorim, I. R.; Leonard, J. A.; Posada, D; Castroviejo, J; Petrucci-Fonseca, F; Crandall, K. A.; Ellegren, H; Wayne, R. K. (1999). "Mitochondrial DNA phylogeography and population history of the grey wolf canis lupus". Molecular Ecology. 8 (12): 2089–103. doi:10.1046/j.1365-294x.1999.00825.x. PMID 10632860. Refer Table 1
- ^ Baryshnikov, Gennady F.; Mol, Dick; Tikhonov, Alexei N (2009). "Finding of the Late Pleistocene carnivores in Taimyr Peninsula (Russia, Siberia) with paleoecological context" (PDF). Russian Journal of Theriology. 8 (2). Russian Journal of Theriology: 107–113. Retrieved 20 May 2017.
- ^ Lacelle, Denis; Lauriol, Bernard; Zazula, Grant; Ghaleb, Bassam; Utting, Nicholas; Clark, Ian D. (2013). "Timing of advance and basal condition of the Laurentide Ice Sheet during the last glacial maximum in the Richardson Mountains, NWT". Quaternary Research. 80 (2): 274. doi:10.1016/j.yqres.2013.06.001.
- ^ Shapiro, B.; Drummond, A. J.; Rambaut, A; Wilson, M. C.; Matheus, P. E.; Sher, A. V.; Pybus, O. G.; Gilbert, M. T.; Barnes, I; Binladen, J; Willerslev, E; Hansen, A. J.; Baryshnikov, G. F.; Burns, J. A.; Davydov, S; Driver, J. C.; Froese, D. G.; Harington, C. R.; Keddie, G; Kosintsev, P; Kunz, M. L.; Martin, L. D.; Stephenson, R. O.; Storer, J; Tedford, R; Zimov, S; Cooper, A (2004). "Rise and Fall of the Beringian Steppe Bison". Science. 306 (5701): 1561–5. doi:10.1126/science.1101074. PMID 15567864.
- ^ Heintzman, Peter D.; Froese, Duane; Ives, John W.; Soares, André E. R.; Zazula, Grant D.; Letts, Brandon; Andrews, Thomas D.; Driver, Jonathan C.; Hall, Elizabeth; Hare, P. Gregory; Jass, Christopher N.; MacKay, Glen; Southon, John R.; Stiller, Mathias; Woywitka, Robin; Suchard, Marc A.; Shapiro, Beth (2016). "Bison phylogeography constrains dispersal and viability of the Ice Free Corridor in western Canada". Proceedings of the National Academy of Sciences. 113 (29): 8057–63. doi:10.1073/pnas.1601077113. PMC 4961175. PMID 27274051.
- ^ Steven M. Stanley (1987). Extinction. Scientific American Library, New York. p. 242.
- ^ O'Keefe, F.R.; Fet, E.V.; Harris, J.M. (2009). "Compilation, calibration, and synthesis of faunal and floral radiocarbon dates, Rancho La Brea, California". Contributions to Science. 518: 1–16.
- ^ Graham, R. W.; Mead, J. I. (1987). "Environmental fluctuations and evolution of mammalian faunas during the last deglaciation in North America". In Ruddiman, W. F.; Wright, H. E. (eds.). North America and Adjacent Oceans During the Last Deglaciation. Geological Society of America K-3, Boulder, Colorado. pp. 371–402. ISBN 978-0-8137-5203-7.
- ^ Barnosky, A. D. (1989). "The Late Pleistocene extinction event as a paradigm for widespread mammal extinction". In Donovan, Stephen K. (ed.). Mass Extinctions: Processes and Evidence. Columbia University Press, New York. pp. 235–255. ISBN 978-0-231-07091-1.
- ^ Brannick, Alexandria L.; Meachen, Julie A.; O'Keefe, F. Robin (September 15, 2015). John M. Harris (ed.). "Microevolution of Jaw Shape in the Dire Wolf, Canis dirus, at Rancho La Brea". Science Series 42. Contributions in Science (A special volume entitled La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas in commemoration of the 100th anniversary of the Natural History Museum of Los Angeles County's excavations at Rancho La Brea). Natural History Museum of Los Angeles County: 23–32.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Cooper, A. (2015). "Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover". Science. 349 (6248): 602–6. doi:10.1126/science.aac4315. PMID 26250679.
- ^ a b Ersmark, Erik; Klütsch, Cornelya F. C.; Chan, Yvonne L.; Sinding, Mikkel-Holger S.; Fain, Steven R.; Illarionova, Natalia A.; Oskarsson, Mattias; Uhlén, Mathias; Zhang, Ya-Ping; Dalén, Love; Savolainen, Peter (2016). "From the Past to the Present: Wolf Phylogeography and Demographic History Based on the Mitochondrial Control Region". Frontiers in Ecology and Evolution. 4. doi:10.3389/fevo.2016.00134.
{{cite journal}}: CS1 maint: unflagged free DOI (link)Refer Page 5 with Table S3, relationship between Clu108(Russia 18,000) and Clu109(Alaska28,000) with Clu8,9,10,22(China). - ^ a b Haile, J.; Froese, D. G.; MacPhee, R. D. E.; Roberts, R. G.; Arnold, L. J.; Reyes, A. V.; Rasmussen, M.; Nielsen, R.; Brook, B. W.; Robinson, S.; Demuro, M.; Gilbert, M. T. P.; Munch, K.; Austin, J. J.; Cooper, A.; Barnes, I.; Moller, P.; Willerslev, E. (2009). "Ancient DNA reveals late survival of mammoth and horse in interior Alaska". Proceedings of the National Academy of Sciences. 106 (52): 22352. Bibcode:2009PNAS..10622352H. doi:10.1073/pnas.0912510106. PMC 2795395. PMID 20018740.
- ^ Pavelková Řičánková, Věra; Robovský, Jan; Riegert, Jan (2014). "Ecological Structure of Recent and Last Glacial Mammalian Faunas in Northern Eurasia: The Case of Altai-Sayan Refugium". PLoS ONE. 9 (1): e85056. doi:10.1371/journal.pone.0085056. PMC 3890305. PMID 24454791.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Beringian wolf mandible dated 31,700 YBP showing large, sharp lower carnassial - Museum of the North, University of Alaska (Arctos database)
- Beringian wolf mandible dated 31,700 YBP - other side view of the specimen above
- Beringian Research Notes - Ancient Northern Wolves Government of the Yukon
- Ice Age Mammals of the Yukon Government of the Yukon
