Gattermann reaction
The Gattermann reaction, (also known as the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by hydrogen cyanide in the presence of a Friedel–Crafts catalyst (e.g. AlCl3). It is named for the German chemist Ludwig Gattermann[1] and is similar to the Friedel-Crafts reaction.
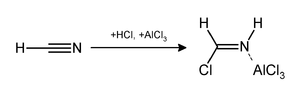

The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide.[2] Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN;[3] additionally, because the reaction uses HCl, Zn(CN)2 also supplies the reaction with ZnCl2 in-situ, where it acts as a Lewis acid catalyst. Examples of Zn(CN)2 being used in this way include the synthesis of 2-Hydroxy-1-nafthaldehyde[2] and Mesitaldehyde.[4]
Gattermann–Koch reaction
The Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch,[5] refers to a Friedel–Crafts acylation reaction in which carbon monoxide, hydrochloric acid, and a Friedel–Crafts catalyst (e.g. AlCl3) are used to produce aromatic aldehydes from various aromatic compounds, including derivatives of benzene and naphthalene:[6]

The applicability of the reaction includes many substituted aromatic derivatives, for example the conversion of toluene (The reaction above displays benzene to p-tolualdehyde) to p-tolualdehyde.[7] However, unlike the Gattermann reaction with HCN, this reaction is not applicable to phenol and phenol ether substrates.[3] Additionally, when Zinc chloride is used as the catalyst, the presence of traces of copper(I) chloride co-catalyst is often necessary.
See also
References
- ^ L. Gattermann; W. Berchelmann (1898). "Synthese aromatischer Oxyaldehyde". Berichte der deutschen chemischen Gesellschaft. 31 (2): 1765–1769. doi:10.1002/cber.18980310281.
- ^ a b Adams R., Levine I. (1923). "Simplification of the Gattermann Synthesis of Hydroxy Aldehydes". J. Am. Chem. Soc. 45 (10): 2373–77. doi:10.1021/ja01663a020.
- ^ a b Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. pp. 38 & 53–54. doi:10.1002/0471264180.or009.02. ISBN 9780471007265.
- ^ Fuson R. C., Horning E. C., Rowland S. P., Ward M. L. (1955). "Mesitaldehyde". Organic Syntheses. doi:10.15227/orgsyn.023.0057
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 549. - ^ Gattermann, L.; Koch, J. A. (1897). "Eine Synthese aromatischer Aldehyde". Ber. 30: 1622. doi:10.1002/cber.18970300288.
- ^ LI Jie Jack (2003). Name Reactions: A Collection of Detailed Reaction Mechanisms (available on Google Books) (2nd ed.). Springer. p. 157. ISBN 3-540-40203-9.
- ^ G. H. Coleman, David Craig (1943). "p-Tolualdehyde". Organic Syntheses; Collected Volumes, vol. 2, p. 583.
