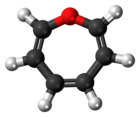
Oxepin
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Oxepine
| |||
| Other names
Oxacycloheptatriene
Benzene oxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6O | |||
| Molar mass | 94.11 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. It exists as an equilibrium mixture with benzene oxide. The oxepin–benzene oxide equilibrium can easily be shifted to one extreme or the other in compounds with this core depending what substituents are present.[1] This compound is not aromatic.
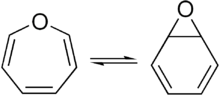
Oxepin is an intermediary in vivo metabolite of benzene in animal models, metabolized by the cytochrome P450 enzyme.[2]
References
Wikimedia Commons has media related to Oxepins.
- ^ E. Vogel and H. Günther (1967). "Benzene Oxide-Oxepin Valence Tautomerism". Angewandte Chemie International Edition in English. 6 (5): 385–401. doi:10.1002/anie.196703851.
- ^ R. Snyder, G. Witz, and B. D. Goldstein (1993). "The toxicology of benzene". Environmental Health Perspectives. 100: 293–306. doi:10.2307/3431535.
{{cite journal}}: CS1 maint: multiple names: authors list (link)