δ34S
The δ34S (pronounced delta 34 S) value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur, 34S:32S, in a sample against the equivalent ratio in a known reference standard. The most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil or per mille, using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfides, have been studied and interpreted as records of the changing sulfur cycle throughout the earth's history.
Calculation
[edit]Of the 25 known isotopes of sulfur, four are stable.[1] In order of their abundance, those isotopes are 32S (94.93%), 34S (4.29%), 33S (0.76%), and 36S (0.02%).[2] The δ34S value refers to a measure of the ratio of the two most common stable sulfur isotopes, 34S:32S, as measured in a sample against that same ratio as measured in a known reference standard. The lowercase delta character is used by convention, to be consistent with use in other areas of stable isotope chemistry.[3] That value can be calculated in per mil (‰, parts per thousand) as:[4]
- ‰
Less commonly, if the appropriate isotope abundances are measured, similar formulae can be used to quantify ratio variations between 33S and 32S, and 36S and 32S, reported as δ33S and δ36S, respectively.[5]
Reference standard
[edit]
Sulfur from meteorites was determined in the early 1950s to be an adequate reference standard because it exhibited a small variability in isotopic ratios.[6] It was also believed that because of their extraterrestrial provenances, meteors represented primordial terrestrial isotopic conditions.[7] During a meeting of the National Science Foundation in April 1962, troilite from the Canyon Diablo meteorite found in Arizona, US, was established as the standard with which δ34S values (and other sulfur stable isotopic ratios) could be calculated.[6][8] Known as Canyon Diablo Troilite (CDT), the standard was established as having a 32S:34S ratio of 22.220 and was used for around three decades.[6] In 1993, the International Atomic Energy Agency (IAEA) established a new standard, Vienna-CDT (VCDT), based on artificially prepared silver sulfide (IAEA-S-1) that was defined to have a δ34SVCDT value of −0.3‰.[8] In 1994, the original CDT material was found not to be isotopically homogeneous, with internal variations as great as 0.4‰, confirming its unsuitability as a reference standard.[6]
Causes of variations
[edit]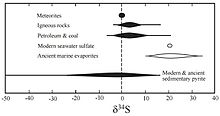
Two mechanisms of fractionation occur that alter sulfur stable isotope ratios: kinetic effects, especially due to the metabolism of sulfate-reducing bacteria, and isotope exchange reactions that occur between sulfide phases based on temperature.[9] With VCDT as the reference standard, natural δ34S value variations have been recorded between −72‰ and +147‰.[10][11]
The presence of sulfate-reducing bacteria, which reduce sulfate (SO2−
4) to hydrogen sulfide (H2S), has played a significant role in the oceanic δ34S value throughout the earth's history. Sulfate-reducing bacteria metabolize 32S more readily than 34S, resulting in an increase in the value of the δ34S in the remaining sulfate in the seawater.[7] Archean pyrite found in barite in the Warrawoona Group, Western Australia, with sulfur fractionations as great as 21.1‰ hint at the presence of sulfate-reducers as early as 3,470 million years ago.[12]
It is now better known that the degree of isotope fractionation during microbial sulfate reduction depends on the cell-specific sulfate reduction rate of the sulfate-reducing microorganism.[13][14] The relative extent of sulfur isotope fractionating activities, including sulfate reduction, sulfide reoxidation and disproportionation, determines the isotopic compositions of the minerals or fluid measured.[15] Other than microbial activities and environmental conditions, isotopic compositions also change due to diffusion, accumulation and mixing after burial.[16][17][15]
The δ34S value, recorded by sulfate in marine evaporites, can be used to chart the sulfur cycle throughout earth's history.[7][4] The Great Oxygenation Event around 2,400 million years ago altered the sulfur cycle radically, as increased atmospheric oxygen permitted an increase in the mechanisms that could fractionate sulfur isotopes, leading to an increase in the δ34S value from ~0‰ pre-oxygenation. Approximately 700 million years ago, the δ34S values in seawater sulfates began to vary more and those in sedimentary sulfates grew more negative. Researchers have interpreted this excursion as indicative of an increase in water column oxygenation with continued periods of anoxia in the deepest waters. Modern seawater sulfate δ34S values are consistently 21.0 ± 0.2‰ across the world's oceans, while sedimentary sulfides vary widely. Seawater sulfate δ34S and δ18O values exhibit similar trends not seen in sedimentary sulfide minerals.[7]
See also
[edit]References
[edit]- ^ Audi G, Bersillon O, Blachot J, Wapstra AH (December 2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. CiteSeerX 10.1.1.692.8504. doi:10.1016/j.nuclphysa.2003.11.001.

- ^ Hoefs 2009, p. 71.
- ^ Cook PG, Herczeg AL, eds. (2000). Environmental Tracers in Subsurface Hydrology (PDF). New York City: Springer Science+Business Media. p. 511. doi:10.1007/978-1-4615-4557-6. ISBN 978-1-4615-4557-6.
- ^ a b Canfield DE (2001). "Biogeochemistry of Sulfur Isotopes". Reviews in Mineralogy and Geochemistry. 43 (1): 607–636. Bibcode:2001RvMG...43..607C. doi:10.2138/gsrmg.43.1.607.

- ^ Whitehouse MJ (March 2013). "Multiple Sulfur Isotope Determination by SIMS: Evaluation of Reference Sulfides for Δ33S with Observations and a Case Study on the Determination of Δ36S". Geostandards and Geoanalytical Research. 37 (1): 19–33. doi:10.1111/j.1751-908X.2012.00188.x. S2CID 95283077.

- ^ a b c d Beaudoin G, Taylor BE, Rumble III D, Thiemens M (October 1994). "Variations in the sulfur isotope composition of troilite from the Cañon Diablo iron meteorite". Geochimica et Cosmochimica Acta. 58 (19): 4253–4255. Bibcode:1994GeCoA..58.4253B. doi:10.1016/0016-7037(94)90277-1.

- ^ a b c d Seal II RR (2006). "Sulfur Isotope Geochemistry of Sulfide Minerals". Reviews in Mineralogy and Geochemistry. 61 (1): 633–677. Bibcode:2006RvMG...61..633S. doi:10.2138/rmg.2006.61.12.

- ^ a b Hoefs 2009, p. 72.
- ^ Hoefs 2009, pp. 73, 77.
- ^ Lever MA, Rouxel O, Alt JC, Shimizu N, Ono S, Coggon RM, et al. (March 2013). "Evidence for microbial carbon and sulfur cycling in deeply buried ridge flank basalt". Science. 339 (6125): 1305–1308. Bibcode:2013Sci...339.1305L. doi:10.1126/science.1229240. PMID 23493710. S2CID 10728606.

- ^ Drake H, Roberts NM, Reinhardt M, Whitehouse M, Ivarsson M, Karlsson A, et al. (June 2021). "Biosignatures of ancient microbial life are present across the igneous crust of the Fennoscandian shield". Communications Earth & Environment. 2 (1): 1–13. doi:10.1038/s43247-021-00170-2.
- ^ Shen Y, Buick R, Canfield DE (March 2001). "Isotopic evidence for microbial sulphate reduction in the early Archaean era". Nature. 410 (6824): 77–81. Bibcode:2001Natur.410...77S. doi:10.1038/35065071. PMID 11242044. S2CID 25375808.

- ^ Wing BA, Halevy I (December 2014). "Intracellular metabolite levels shape sulfur isotope fractionation during microbial sulfate respiration". Proceedings of the National Academy of Sciences of the United States of America. 111 (51): 18116–18125. Bibcode:2014PNAS..11118116W. doi:10.1073/pnas.1407502111. PMC 4280625. PMID 25362045.
- ^ Wenk CB, Wing BA, Halevy I (October 2017). "Electron carriers in microbial sulfate reduction inferred from experimental and environmental sulfur isotope fractionations". The ISME Journal. 12 (2): 495–507. doi:10.1038/ismej.2017.185. PMC 5776465. PMID 29087380.
- ^ a b Tsang MY, Wortmann UG (July 2022). "Sulfur isotope fractionation derived from reaction-transport modelling in the Eastern Equatorial Pacific". Journal of the Geological Society. 179 (5). Bibcode:2022JGSoc.179...68T. doi:10.1144/jgs2021-068. ISSN 0016-7649. S2CID 248647580.
- ^ Jørgensen BB (January 1978). "A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments: II. Calculation from mathematical models". Geomicrobiology Journal. 1 (1): 29–47. doi:10.1080/01490457809377722. ISSN 0149-0451.
- ^ Berner RA (2020). Early Diagenesis: A Theoretical Approach. Princeton, NJ: Princeton University Press. doi:10.1515/9780691209401. ISBN 978-0-691-20940-1. OCLC 1164642477.
Sources
[edit]- Hoefs J (2009). Stable Isotope Geochemistry (6th ed.). Berlin: Springer-Verlag. doi:10.1007/978-3-540-70708-0. ISBN 978-3-540-70703-5.

