1,2-Wittig rearrangement
A 1,2-Wittig rearrangement is a categorization of chemical reactions in organic chemistry, and consists of a 1,2-rearrangement of an ether with an alkyllithium compound.[1][2] The reaction is named for Nobel Prize winning chemist Georg Wittig.
The intermediate product is an alkoxy lithium salt and the final product an alcohol. When R2 is a good leaving group and electron withdrawing functional group such as a cyanide (CN) group,[3] this group is eliminated and the corresponding ketone is formed.
Reaction mechanism
The reaction mechanism centers on the formation of a free radical pair with lithium migrating from the carbon atom to the oxygen atom. The R radical then recombines with the ketyl.[4]
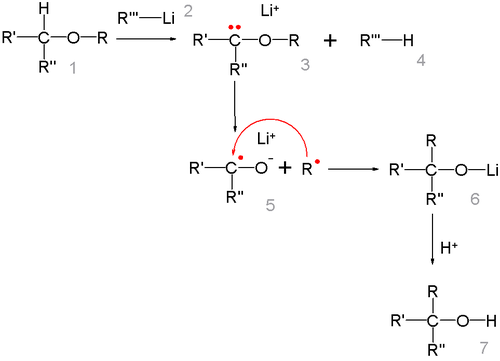
The alkyl group migrates in the order of thermodynamical stability methyl < primary alkyl < secondary alkyl < tertiary alkyl in this is line with the radical mechanism. The radical-ketyl pair is short lived and due to a solvent cage effect some isomerizations take place with retention of configuration.
With certain allyl aryl ethers a competing reaction mechanism takes place.[4] The reaction of allyl phenyl ether 1 with sec-butyllithium at −78 °C gives the lithiated intermediate 2 which on heating to −25 °C only shows the rearranged product 5 but not 4 after trapping the lithium alkoxide with trimethylsilyl chloride. This result rules out a radical-ketyl intermediate 3a in favor of the Meisenheimer complex 3b. Additional evidence for this mechanism is provided by the finding that with a para tert-butyl substituent the reaction is retarded.

The reaction is a formal dyotropic reaction.
See also
References
- ^ Georg Wittig, L. Löhmann, Ann. 550, 260 (1942)
- ^ G. Wittig, Experientia 14, 389 (1958).
- ^ Preparation of aryl benzyl ketones by [1,2]-Wittig rearrangement Alan R. Katritzky, Yuming Zhang, Sandeep K. Singh Arkivoc pp. 146–50 2002 (vii) link
- ^ a b Wittig Rearrangement of Lithiated Allyl Aryl Ethers: A Mechanistic Study Sven Strunk, Manfred Schlosser European Journal of Organic Chemistry Volume 2006, Issue 19 , pp. 4393–97 doi:10.1002/ejoc.200600304
