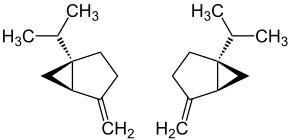
Sabinene
Appearance
| |
| Names | |
|---|---|
| IUPAC name
4-methylene-1-(1-methylethyl)bicyclo[3.1.0]hexane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.194 |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.23 g/mol |
| Density | 0.844 g/mL at 20 °C g/cm3 |
| Boiling point | 163 to 164 °C (325 to 327 °F; 436 to 437 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including holm oak (Quercus ilex) and Norway spruce (Picea abies). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring.
Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg,[2] Laurus nobilis, and Clausena anisata.
See also
- Thujene, a double bond isomer of sabinene
