Racetam


Racetams are a class of drugs that share a pyrrolidone nucleus.[1] Some, such as piracetam, aniracetam, oxiracetam, pramiracetam and phenylpiracetam are considered nootropics.[2] Others such as levetiracetam, brivaracetam, and seletracetam are anticonvulsants.
Mechanism
There is no universally accepted mechanism of action for racetams. Racetams generally show negligible affinity for common central nervous system receptors, but modulation of central neurotransmitters, including acetylcholine and glutamate, has been reported. Although aniracetam and nebracetam show affinity for muscarinic receptors, only nefiracetam demonstrates nanomolar interactions. Modification of membrane-located mechanisms of central signal transduction is another hypothesis.[3]
Like some ampakines, some racetams such as piracetam and aniracetam are positive allosteric modulators of the AMPA receptor.[4]
Racetams are understood to work by allosterically modulating glutamate receptors, specifically AMPA receptors, leading to Ca2+ influx that is excitatory.[5] Racetams are posited to enhance memory through interaction with glutamate receptors in the central nervous system.
Methylphenylpiracetam is a positive allosteric modulator of the sigma-1 receptor.[6]
Cofactors
In studies with aged rats, marked improvement has been observed in cognitive tasks in experimental groups given piracetam. Performance was further increased when piracetam was combined with choline. Evidence in studies with rats has indicated that the potency of piracetam is increased when administered with choline.[7]
List of Racetams
| Structure | Name |
|---|---|
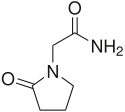 |
Piracetam |
 |
Oxiracetam |
 |
Phenylpiracetam / Fonturacetam |
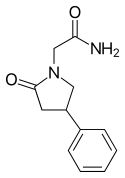
 |
Phenylpiracetam Hydrazide / Fonturacetam Hydrazide |
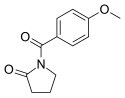 |
Aniracetam |
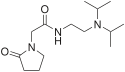 |
Pramiracetam |
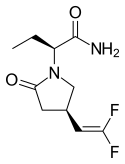 |
Seletracetam |
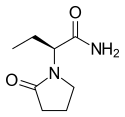 |
Levetiracetam |
 |
Coluracetam / BCI-540 |
 |
Fasoracetam |
 |
Brivaracetam |
 |
Dimiracetam |
 |
Methylphenylpiracetam / E1R |
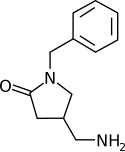 |
Nebracetam |
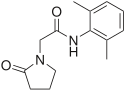 |
Nefiracetam |
 |
Omberacetam / Noopept |
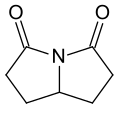 |
Rolziracetam |
 |
Cebaracetam |
Legality
Australia
All racetams are schedule 4 substances in Australia under the Poisons Standard (February 2020).[8] A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription."[8]
References
- ^ Löscher W, Richter A (March 2000). "Piracetam and levetiracetam, two pyrrolidone derivatives, exert antidystonic activity in a hamster model of paroxysmal dystonia". European Journal of Pharmacology. 391 (3): 251–4. doi:10.1016/S0014-2999(00)00105-9. PMID 10729365.
- ^ Cohen, Pieter A.; Zakharevich, Igor; Gerona, Roy (25 November 2019). "Presence of Piracetam in Cognitive Enhancement Dietary Supplements". JAMA Internal Medicine. doi:10.1001/jamainternmed.2019.5507. PMC 6902196. PMID 31764936.
- ^ Gualtieri F, Manetti D, Romanelli MN, Ghelardini C (2002). "Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs". Current Pharmaceutical Design. 8 (2): 125–38. doi:10.2174/1381612023396582. PMID 11812254.
- ^ Ahmed AH, Oswald RE (March 2010). "Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors". Journal of Medicinal Chemistry. 53 (5): 2197–203. doi:10.1021/jm901905j. PMC 2872987. PMID 20163115.
- ^ Copani A, Genazzani AA, Aleppo G, Casabona G, Canonico PL, Scapagnini U, Nicoletti F (April 1992). "Nootropic drugs positively modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-sensitive glutamate receptors in neuronal cultures". Journal of Neurochemistry. 58 (4): 1199–204. doi:10.1111/j.1471-4159.1992.tb11329.x. PMID 1372342.
- ^ Vavers E, Zvejniece L, Veinberg G, Svalbe B, Domracheva I, Vilskersts R, Dambrova M (2015). "Novel positive allosteric modulators of sigma-1 receptor". SpringerPlus. 4: P51. doi:10.1186/2193-1801-4-S1-P51. PMC 4797911.
The R-configuration enantiomers of methylphenylpiracetam are more active positive allosteric modulators of Sigma-1 receptor than S-configuration enantiomers.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bartus RT, Dean RL, Sherman KA, Friedman E, Beer B (1981). "Profound effects of combining choline and piracetam on memory enhancement and cholinergic function in aged rats". Neurobiology of Aging. 2 (2): 105–11. doi:10.1016/0197-4580(81)90007-5. PMID 7301036.
- ^ a b Poisons Standard February 2020. comlaw.gov.au
