Alkannin
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
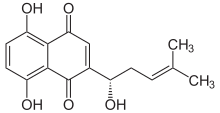
5,8-Dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione | |
| Other names
C.I. Natural red 20
Alkanet extract Anchusaic acid Anchusin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.497 |
| E number | E103 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C16H16O5 | |
| Molar mass | 288.299 g·mol−1 |
| Appearance | Red-brown crystalline prisms |
| Density | 1.15 g/mL |
| Melting point | 149 °C (300 °F; 422 K) |
| Boiling point | 567 °C (1,053 °F; 840 K) |
| Sparingly soluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3.0 g/kg (mice) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alkannin is a natural dye that is obtained from the extracts of plants from the borage family Alkanna tinctoria that are found in the south of France. The dye is used as a food colouring and in cosmetics. It is used as a red-brown food additive in regions such as Australia.[2] Alkannin is deep red in an acid and blue in an alkaline environment.[3] The chemical structure as a naphthoquinone derivative was first determined by Brockmann in 1936.[4] The R-enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.[5][6]
Biosynthesis
The enzyme 4-hydroxybenzoate geranyltransferase utilises geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used to form alkannin.[6]
Research
Because the root bark (cork layers) of this plant contains large amounts of red naphthoquinone pigments, the roots of these plants are red-purple. If shikonin is extracted from fresh tissues, it gradually darkens over several days, finally forming black precipitates, which are thought to be polymers.[7]
References
- ^ The Merck Index, 11th Edition, 243
- ^ Additives Archived 2011-04-06 at the Wayback Machine, Food Standards Australia New Zealand
- ^ "Alkanet" in Dispensatory of the United States of America, year 1918, edited by Joseph P. Remington and Horatio C. Wood.
- ^ H. Brockmann (1936). "Die Konstitution des Alkannins, Shikonins und Alkannans". Justus Liebigs Ann. Chem. 521: 1–47. doi:10.1002/jlac.19365210102.
- ^ Shmuel Yannai (2012). Dictionary of Food Compounds. CRC Press. p. 478.
- ^ a b Vassilios P. Papageorgiou; Andreana N. Assimopoulou; Elias A. Couladouros; et al. (1999). "The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products". Angew. Chem. Int. Ed. 38 (3): 270–300. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0.
- ^ Kazufumi Y. (2017). Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnology 34: 131–142.
