Kairine
Appearance
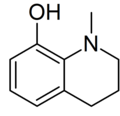
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Methyl-1,2,3,4-tetrahydroquinolin-8-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13NO | |
| Molar mass | 163.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Kairine is a derivative of tetrahydroquinoline which was first described by Wilhelm Fischer in 1883. Its name comes from the Greek kairos, meaning "the right time".[1] It is an antipyretic, formerly used against typhoid fever, but now largely obsolete due to severe side effects. Both kairine and its N-ethyl homolog show similar antipyretic activity.[2][3][4][5]
See also
[edit]References
[edit]- ^ W.E.Flood (1963). The Origins of Chemical Names. Oldbourne Book Co Ltd. p. 126.
- ^ Fischer, Wilhelm (1883). "On Kairine and Kairoline". New Remedies. 12 (2): 41.
- ^ Fruitnight, J. Henry (1886). "Kairine and Antipyrine". Medical Record. 29 (23): 646–648.
- ^ Bockmuhl M, Dorzbach E. Antipyretics of the tetrahydroquinoline series. Med. u. Chem. (1942) 4: 179-212.
- ^ Slater, Leo Barney (2009). War and Disease: Biomedical Research on Malaria in the Twentieth Century. Rutgers University Press. p. 26. ISBN 978-0-8135-4438-0.
