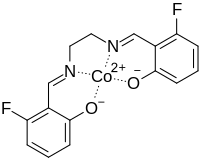
Fluomine
Appearance
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.057.672 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12CoF2N2O2 | |
| Molar mass | 361.214 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluomine is a chemical compound containing a cobalt chelate. It has the ability to form a complex with molecular oxygen (O2) and then release it upon heating.[1] Because of this ability to reversibly sorb and desorb oxygen, it has been used in high-altitude aircraft oxygen-generating systems.[2][3]
The toxicity of fluomine has been studied[1][4] and it is classified by the Emergency Planning and Community Right-to-Know Act as an extremely hazardous substance.[5]
References
[edit]- ^ a b Kinkead, E.R.; Haun, C.C.; Bowers, R.S.; Vernot, E.H.; Mac Ewen, J.D.; Amster, R.L. (1981). "The mammalian toxicity of fluomine dust". American Industrial Hygiene Association Journal. 42 (9): 675–680. doi:10.1080/15298668191420503. PMID 7293930.
- ^ Manatt, S. A. (1981). "Onboard oxygen generation systems". Aviation, Space, and Environmental Medicine. 52 (11 Pt 1): 645–53. PMID 7305791.
- ^ "Breathing Oxygen: Purity of Oxygen Generated By a Fulomine-Based System" (PDF). USAF School of Aerospace Medicine, Aerospace Medical Division (AFSC). September 1976. Archived (PDF) from the original on June 1, 2022. Retrieved March 10, 2019.
- ^ Kinkead, E.R.; Haun, C.C.; Bowers, R.S.; Vernot, E.H.; MacEwen, J.D.; Amster, R.L. (1982). "Six month inhalation toxicity of fluomine dust". American Industrial Hygiene Association Journal. 43 (1): 66–71. doi:10.1080/15298668291409361. PMID 7055087.
- ^ 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (PDF) (July 1, 2008 ed.), Government Printing Office, archived from the original (PDF) on 2012-02-25, retrieved March 8, 2009
