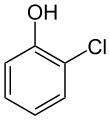
2-Chlorophenol
Appearance
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chlorophenol[1] | |||
| Other names
o-Chlorophenol
ortho-Chlorophenol 2-Hydroxychlorobenzene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.213 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H5ClO | |||
| Molar mass | 128.56 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 1.2634 g/cm3 at 20 °C[2] | ||
| Melting point | 8 °C (46 °F; 281 K)[2] | ||
| Boiling point | 173.4 °C (344.1 °F; 446.5 K)[2] | ||
| 20 g/L at 20 °C | |||
| Solubility | Soluble in ethanol, diethyl ether, benzene | ||
| Vapor pressure | 0.308 kPa[3] | ||
| Acidity (pKa) | 8.56[4] | ||
| -77.3·10−6 cm3/mol[5] | |||
Refractive index (nD)
|
1.5524[2] | ||
| Viscosity | 3.59 mPa·s[6] | ||
| Thermochemistry[7] | |||
Heat capacity (C)
|
188.7 J·mol−1·K−1 | ||
Enthalpy of fusion (ΔfH⦵fus)
|
13.0 kJ·mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive – causes burns | ||
| Flash point | 64 °C (147 °F; 337 K)[6] | ||
| 550 °C (1,022 °F; 823 K) | |||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related aromatic
hydrocarbons |
benzene phenol chlorobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
2-Chlorophenol or ortho-chlorophenol is an organic compound with the formula C6H4ClOH. It is one of three isomeric monochloride derivatives of phenol. As from occasional use as a disinfectant, it has few applications. It is an intermediate in the polychlorination of phenol.[8] 2-Chlorophenol is a colorless liquid, although commercial samples are often yellow or amber-colored. It has an unpleasant, penetrating (carbolic) odor. It is poorly soluble in water.
See also
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
- ^ a b c d Haynes, p. 3.116
- ^ Haynes, p. 16.20
- ^ Haynes, p. 5.90
- ^ Haynes, p. 3.577
- ^ a b Haynes, p. 6.243
- ^ Haynes, pp. 5.10, 6.156
- ^ Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External links
- ToxFAQs for Chlorophenols, Agency for Toxic Substances and Disease Registry.
- Compound Summary Compendium, PubChem Open Chemistry Database.