Mukaiyama hydration
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.[1]
The reaction was developed by Teruaki Mukaiyama at Mitsui Petrochemical Industries, Ltd. Its discovery was based on previous work on the selective hydrations of olefins catalyzed by cobalt complexes with Schiff base ligands[2] and porphyrin ligands.[3] Due to its chemoselectivity (tolerant of other functional groups) and mild reactions conditions (run under air at room temperature), the Mukaiyama hydration has become a valuable tool in chemical synthesis.
Mechanism
In his original publication, Mukaiyama proposed that the reaction proceeded through the intermediacy of a cobalt peroxide adduct. A metal exchange reaction between a hydrosilane and the cobalt peroxide adduct leads to a silyl peroxide, which is converted to the alcohol upon reduction, presumably via action of the cobalt catalyst.

Studies investigating the mechanism of cobalt-catalyzed peroxidation of alkenes by Nojima and coworkers,[4] support the intermediacy of a metal hydride that reacts with the alkene directly to form a transient cobalt-alkyl bond. Homolysis generates a carbon centered radical that reacts directly with oxygen and is subsequently trapped by a cobalt(II) species to form the same cobalt-peroxide adduct as suggested by Mukaiyama. Metal exchange with the hydrosilane produces a silyl peroxide product and further reduction (via homolysis of the oxygen-oxygen bond) leads to the product alcohol. The use of a silane reductant allows for this reaction to be carried out without heat.[5] The authors also note, in accordance with previous studies,[6] that the addition of t-butylhydroperoxide can increase the rate of slower-reacting substrates. This rate increase is likely due to oxidation of cobalt(II) to alkylperoxo-cobalt(III) complex, which subsequently participates in a rapid metal exchange with the hydrosilane to generate the active cobalt(III)-hydride.

It is important to note that the mechanism laid out above is in marked contrast to previous mechanistic proposals,[7] which suggest that a cobalt-peroxy complex inserts directly into alkenes. The aforementioned study by Nojima and coworkers disagrees with this proposal due to three observations: 1) the intermediacy of a cobalt-hydride observed via 1H NMR 2) the propensity of alkenes to undergo autooxidation to the α, β-unsaturated ketones or allylic alcohols when the same reaction is run in the absence of a hydrosilane 3) the predominant mode of decomposition of alkylperoxo-cobalt(III) species to an alkoxy or alkylperoxy radical via the Haber–Weiss mechanism.
A recent review by Shenvi and coworkers,[8] proposed that the Mukaiyama hydration operates via the same principles as metal hydride hydrogen atom transfer (MH HAT), elucidated by Jack Halpern and Jack R. Norton in their studies on hydrogenation of anthracenes by syngas and Co2(CO)8[9] and the chemistry of vitamin B12 mimics,[10] respectively.
Variations
Carbon-oxygen bond formation
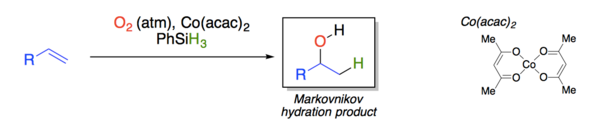
Yamada explored the effect of different solvents and cobalt beta-diketonate ligands on the yield and product distribution of the reaction.[11]

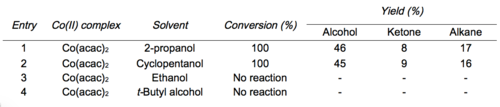
Mukaiyama and Isayama developed conditions to isolate the intermediate silylperoxide.[6][12] Treatment of the intermediate silylperoxide with 1 drop of concentrated HCl in methanol leads to the hydroperoxide product.
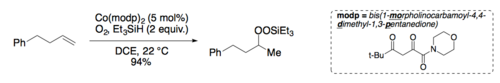
Both Mukaiyama[13] and Magnus[14] describe conditions for an α-enone hydroxylation reaction using Mn(dpm)x in the presence of oxygen and phenylsilane. An asymmetric variant was described by Yamada and coworkers.[15]

Dale Boger and coworkers used a variant of the Mukaiyama hydration, utilizing an iron oxalate catalyst (Fe2ox3•6H2O) in the presence of air, for the total synthesis of vinblastine and related analogs.[16]
Carbon-nitrogen bond formation
Erick Carreira’s group has developed both cobalt and manganese-catalyzed methods for the hydrohydrazination of olefins.[17][18]

Both Carreira[19] and Boger[20] have developed hydroazidation reactions.
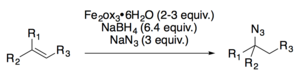
Applications
In total synthesis
The Mukaiyama hydration or variants thereof have been featured in the syntheses of (±)-garsubellin A,[21] stigmalone,[22] vinblastine,[23] (±)-cortistatin A,[24] (±)-lahadinine B,[25] ouabagenin,[26] pectenotoxin-2,[27] (±)-indoxamycin B,[28] trichodermatide A,[29] (+)-omphadiol[30] and many more natural products.
In the following diagram, an application of the Mukaiyama hydration in the total synthesis of (±)-garsubellin A is illustrated:

The hydration reaction is catalyzed by Co(acac)2 (acac = 2,4-pentanedionato, better known as acetylacetonato) and carried out in the presence of air oxygen & phenylsilane. With isopropanol used as solvent, yields of 73 % are obtained.
See also
References
- ^ Isayama, Shigeru; Mukaiyama, Teruaki (1 June 1989). "A New Method for Preparation of Alcohols from Olefins with Molecular Oxygen and Phenylsilane by the Use of Bis(acetylacetonato)cobalt(II)". Chemistry Letters. 18 (6): 1071–1074. doi:10.1246/cl.1989.1071. ISSN 0366-7022.
- ^ Hamilton, Dorothy E.; Drago, Russell S.; Zombeck, Alan (1 January 1987). "Mechanistic studies on the cobalt(II) Schiff base catalyzed oxidation of olefins by O2". Journal of the American Chemical Society. 109 (2): 374–379. doi:10.1021/ja00236a014. ISSN 0002-7863.
- ^ Okamoto, Tadashi; Oka, Shinzaburo (1 May 1984). "Oxygenation of olefins under reductive conditions. Cobalt-catalyzed selective conversion of aromatic olefins to benzylic alcohols by molecular oxygen and tetrahydroborate". The Journal of Organic Chemistry. 49 (9): 1589–1594. doi:10.1021/jo00183a020. ISSN 0022-3263.
- ^ Tokuyasu, Takahiro; Kunikawa, Shigeki; Masuyama, Araki; Nojima, Masatomo (1 October 2002). "Co(III)−Alkyl Complex- and Co(III)−Alkylperoxo Complex-Catalyzed Triethylsilylperoxidation of Alkenes with Molecular Oxygen and Triethylsilane". Organic Letters. 4 (21): 3595–3598. doi:10.1021/ol0201299. ISSN 1523-7060. PMID 12375896.
- ^ Zweig, Joshua E.; Kim, Daria E.; Newhouse, Timothy R. (2017-09-27). "Methods Utilizing First-Row Transition Metals in Natural Product Total Synthesis". Chemical Reviews. 117 (18): 11680–11752. doi:10.1021/acs.chemrev.6b00833. ISSN 0009-2665. PMID 28525261.
- ^ a b Isayama, Shigeru; Mukaiyama, Teruaki (1 April 1989). "Novel Method for the Preparation of Triethylsilyl Peroxides from Olefins by the Reaction with Molecular Oxygen and Triethylsilane Catalyzed by Bis(1,3-diketonato)cobalt(II)". Chemistry Letters. 18 (4): 573–576. doi:10.1246/cl.1989.573. ISSN 0366-7022.
- ^ Hamilton, Dorothy E.; Drago, Russell S.; Zombeck, Alan (1987). "Mechanistic studies on the cobalt(II) Schiff base catalyzed oxidation of olefins by O2". Journal of the American Chemical Society. 109 (2): 374–379. doi:10.1021/ja00236a014.
- ^ Crossley, Steven W. M.; Obradors, Carla; Martinez, Ruben M.; Shenvi, Ryan A. (10 August 2016). "Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins". Chemical Reviews. 116 (15): 8912–9000. doi:10.1021/acs.chemrev.6b00334. PMC 5872827. PMID 27461578.
- ^ Halpern, J. (1 January 1986). "Free radical mechanisms in organometallic and bioorganometallic chemistry". Pure and Applied Chemistry. 58 (4): 575–584. doi:10.1351/pac198658040575. ISSN 0033-4545. S2CID 53710591.
- ^ Estes, Deven P.; Grills, David C.; Norton, Jack R. (17 December 2014). "The Reaction of Cobaloximes with Hydrogen: Products and Thermodynamics". Journal of the American Chemical Society. 136 (50): 17362–17365. doi:10.1021/ja508200g. ISSN 0002-7863. OSTI 1183829. PMID 25427140.
- ^ Kato, Koji; Yamada, Tohru; Takai, Toshihiro; Inoki, Satoshi; Isayama, Shigeru (January 1990). "Catalytic Oxidation–Reduction Hydration of Olefin with Molecular Oxygen in the Presence of Bis(1,3-diketonato)cobalt(II) Complexes". Bulletin of the Chemical Society of Japan. 63 (1): 179–186. doi:10.1246/bcsj.63.179.
- ^ Isayama, Shigeru (1 May 1990). "An Efficient Method for the Direct Peroxygenation of Various Olefinic Compounds with Molecular Oxygen and Triethylsilane Catalyzed by a Cobalt(II) Complex". Bulletin of the Chemical Society of Japan. 63 (5): 1305–1310. doi:10.1246/bcsj.63.1305. ISSN 0009-2673.
- ^ Inoki, Satoshi; Kato, Koji; Isayama, Shigeru; Mukaiyama, Teruaki (1 October 1990). "A New and Facile Method for the Direct Preparation of α-Hydroxycarboxylic Acid Esters from α,β-Unsaturated Carboxylic Acid Esters with Molecular Oxygen and Phenylsilane Catalyzed by Bis(dipivaloylmethanato)manganese(II) Complex". Chemistry Letters. 19 (10): 1869–1872. doi:10.1246/cl.1990.1869. ISSN 0366-7022.
- ^ Magnus, Philip; Payne, Andrew H; Waring, Michael J; Scott, David A; Lynch, Vince (9 December 2000). "Conversion of α,β-unsaturated ketones into α-hydroxy ketones using an MnIII catalyst, phenylsilane and dioxygen: acceleration of conjugate hydride reduction by dioxygen". Tetrahedron Letters. 41 (50): 9725–9730. doi:10.1016/S0040-4039(00)01727-5.
- ^ Sato, Mitsuo; Gunji, Yasuhiko; Ikeno, Taketo; Yamada, Tohru (11 September 2004). "Stereoselective Preparation of α-Hydroxycarboxamide by Manganese Complex Catalyzed Hydration of α,β-Unsaturated Carboxamide with Molecular Oxygen and Phenylsilane". Chemistry Letters. 33 (10): 1304–1305. doi:10.1246/cl.2004.1304. ISSN 0366-7022.
- ^ Ishikawa, Hayato; Colby, David A.; Seto, Shigeki; Va, Porino; Tam, Annie; Kakei, Hiroyuki; Rayl, Thomas J.; Hwang, Inkyu; Boger, Dale L. (8 April 2009). "Total Synthesis of Vinblastine, Vincristine, Related Natural Products, and Key Structural Analogues". Journal of the American Chemical Society. 131 (13): 4904–4916. doi:10.1021/ja809842b. ISSN 0002-7863. PMC 2727944. PMID 19292450.
- ^ Waser, Jérôme; González-Gómez, José C.; Nambu, Hisanori; Huber, Pascal; Carreira, Erick M. (1 September 2005). "Cobalt-Catalyzed Hydrohydrazination of Dienes and Enynes: Access to Allylic and Propargylic Hydrazides". Organic Letters. 7 (19): 4249–4252. doi:10.1021/ol0517473. ISSN 1523-7060. PMID 16146399.
- ^ Waser, Jérôme; Carreira, Erick M. (6 August 2004). "Catalytic Hydrohydrazination of a Wide Range of Alkenes with a Simple Mn Complex". Angewandte Chemie International Edition. 43 (31): 4099–4102. doi:10.1002/anie.200460811. ISSN 1521-3773. PMID 15300706.
- ^ Waser, Jérôme; Nambu, Hisanori; Carreira, Erick M. (1 June 2005). "Cobalt-Catalyzed Hydroazidation of Olefins: Convenient Access to Alkyl Azides". Journal of the American Chemical Society. 127 (23): 8294–8295. doi:10.1021/ja052164r. ISSN 0002-7863. PMID 15941257.
- ^ Leggans, Erick K.; Barker, Timothy J.; Duncan, Katharine K.; Boger, Dale L. (16 March 2012). "Iron(III)/NaBH4-Mediated Additions to Unactivated Alkenes: Synthesis of Novel 20′-Vinblastine Analogues". Organic Letters. 14 (6): 1428–1431. doi:10.1021/ol300173v. ISSN 1523-7060. PMC 3306530. PMID 22369097.
- ^ Kuramochi, Akiyoshi; Usuda, Hiroyuki; Yamatsugu, Kenzo; Kanai, Motomu; Shibasaki, Masakatsu (1 October 2005). "Total Synthesis of (±)-Garsubellin A". Journal of the American Chemical Society. 127 (41): 14200–14201. doi:10.1021/ja055301t. ISSN 0002-7863. PMID 16218611.
- ^ Enders, Dieter; Ridder, André (1 January 2000). "First Asymmetric Synthesis of Stigmolone: The Fruiting Body Inducing Pheromone of the Myxobacterium Stigmatella Aurantiaca". Synthesis. 2000 (13): 1848–1851. doi:10.1055/s-2000-8219. ISSN 0039-7881. S2CID 196825043.
- ^ Ishikawa, Hayato; Colby, David A.; Seto, Shigeki; Va, Porino; Tam, Annie; Kakei, Hiroyuki; Rayl, Thomas J.; Hwang, Inkyu; Boger, Dale L. (8 April 2009). "Total Synthesis of Vinblastine, Vincristine, Related Natural Products, and Key Structural Analogues". Journal of the American Chemical Society. 131 (13): 4904–4916. doi:10.1021/ja809842b. ISSN 0002-7863. PMC 2727944. PMID 19292450.
- ^ Shenvi, Ryan A.; Guerrero, Carlos A.; Shi, Jun; Li, Chuang-Chuang; Baran, Phil S. (1 June 2008). "Synthesis of (+)-Cortistatin A". Journal of the American Chemical Society. 130 (23): 7241–7243. doi:10.1021/ja8023466. ISSN 0002-7863. PMC 2652360. PMID 18479104.
- ^ Magnus, Philip; Westlund, Neil (2 December 2000). "Synthesis of (±)-lahadinine B and (±)-11-methoxykopsilongine". Tetrahedron Letters. 41 (49): 9369–9372. doi:10.1016/S0040-4039(00)01399-X.
- ^ Renata, Hans; Zhou, Qianghui; Baran, Phil S. (4 January 2013). "Strategic Redox Relay Enables A Scalable Synthesis of Ouabagenin, A Bioactive Cardenolide". Science. 339 (6115): 59–63. Bibcode:2013Sci...339...59R. doi:10.1126/science.1230631. ISSN 0036-8075. PMC 4365795. PMID 23288535.
- ^ Bondar, Dmitriy; Liu, Jian; Müller, Thomas; Paquette, Leo A. (1 April 2005). "Pectenotoxin-2 Synthetic Studies. 2. Construction and Conjoining of ABC and DE Eastern Hemisphere Subtargets". Organic Letters. 7 (9): 1813–1816. doi:10.1021/ol0504291. ISSN 1523-7060. PMID 15844913.
- ^ Jeker, Oliver F.; Carreira, Erick M. (2 April 2012). "Total Synthesis and Stereochemical Reassignment of (±)-Indoxamycin B". Angewandte Chemie International Edition. 51 (14): 3474–3477. doi:10.1002/anie.201109175. ISSN 1521-3773. PMID 22345071.
- ^ Shigehisa, Hiroki; Suwa, Yoshihiro; Furiya, Naho; Nakaya, Yuki; Fukushima, Minoru; Ichihashi, Yusuke; Hiroya, Kou (25 March 2013). "Stereocontrolled Synthesis of Trichodermatide A". Angewandte Chemie International Edition. 52 (13): 3646–3649. doi:10.1002/anie.201210099. ISSN 1521-3773. PMID 23417860.
- ^ Liu, Gang; Romo, Daniel (8 August 2011). "Total Synthesis of (+)-Omphadiol". Angewandte Chemie International Edition. 50 (33): 7537–7540. doi:10.1002/anie.201102289. ISSN 1521-3773. PMID 21761524.
