Sulfine
Appearance
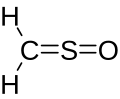
| |
| Names | |
|---|---|
| IUPAC name
sulfinylmethane
| |
| Other names
sulfine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH2OS | |
| Molar mass | 62.09 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfinylmethane or sulfine is an organic compound with molecular formula H2CSO. It is the simplest sulfine. Sulfines are chemical compounds with the general structure XY=SO.[1] IUPAC considers the term 'sulfine' obsolete,[2] preferring instead thiocarbonyl S-oxide, despite this the use of the term sulfine still predominates in the chemical literature.
Substituted sulfines
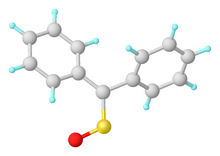
The parent sulfine H2CSO is very labile, whereas substituted derivatives are more conveniently isolated. One example is syn-propanethial-S-oxide which is produced from Allicin and is responsible for eye-watering effects of cutting onions. Another example is diphenylsulfine, obtained by oxidation of thiobenzophenone:[3]
- (C6H5)2C=S + [O] → (C6H5)2C=S=O
See also
- Sulfene - related functional group with the formula H2C=SO2
- Ethenone
- heterocumulene
References
- ^ Binne Zwanenburg (1989). "Sulfine Chemistry". Phosphorus, Sulfur, and Silicon and the Related Elements. 43. doi:10.1080/10426508908040276.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfines". doi:10.1351/goldbook.S06108
- ^ "The crystal and molecular structures of the thiobenzophenone S-oxide and thiobenzophenone". Acta Crystallogr. B35: 1179–1182. 1979. doi:10.1107/S0567740879005835.
{{cite journal}}: Cite uses deprecated parameter|authors=(help)
