Aurantiamine
Appearance
| |
| Names | |
|---|---|
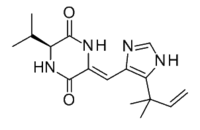
| IUPAC name
(3Z,6S)-3-[[5-(2-Methylbut-3-en-2-yl)-1H-imidazol-4-yl]methylidene]-6-propan-2-ylpiperazine-2,5-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H22N4O2 | |
| Molar mass | 302.378 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
(−)-Aurantiamine is a blue fluorescence metabolite produced by the fungus Penicillium aurantiogriseum, the most common fungi found in cereals.[1] (−)-Aurantiamine belongs to a class of naturally occurring 2,5-diketopiperazines featuring a dehydrohistidine residue that exhibit important biological activities, such as anti-cancer or neurotoxic effects.[2] It is the isopropyl analog of the microtubule binding agent (−)-phenylahistin but is 40 times less active than the latter on P388 cell proliferation.[3] The total asymmetric synthesis of (−)-aurantiamine has been described.[4]
References
- ^ Frisvad JC, Filtenborg O (1989). "Terverticillate penicillia: chemotaxonomy and mycotoxin production". Mycologia. 81 (6): 837–861. doi:10.2307/3760103. JSTOR 3760103.
- ^ Borthwick AD (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ^ Larsen TO, Frisvad JC, Jensen SR (1992). "Aurantiamine, a diketopiperazine from two varieties of Penicillium aurantiogriseum". Phytochemistry. 31 (5): 1613–1615. doi:10.1016/0031-9422(92)83116-G.
- ^ Couladouros EA, Magos AD (2005). "Total asymmetric synthesis of (–)-Phenylhistine, (–)-Aurantiamine and related compounds. Part I". Molecular Diversity. 9 (1–3): 99–109. doi:10.1007/s11030-005-1294-x. PMID 15789557.
