Castro–Stephens coupling
| Castro–Stephens coupling | |
|---|---|
| Named after | Charles E. Castro Robert D. Stephens |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000525 |
The Castro–Stephens coupling is a cross coupling reaction between a copper(I) acetylide and an aryl halide in pyridine, forming a disubstituted alkyne and a copper(I) halide.[1][2]
The reaction was discovered in 1963 by University of California, Riverside chemists Castro and Stephens[1][2] and is used as a tool in the organic synthesis of organic compounds. The reaction has similarities with the much older Rosenmund–von Braun synthesis (1914)[3][4] between aryl halides and copper(I) cyanide and was itself modified in 1975 with as the Sonogashira coupling by adding a palladium catalyst and preparing the organocopper compound in situ, allowing copper to also be used catalytically.[5][6]
A typical reaction is the coupling of iodobenzene with the copper acetylide of phenylacetylene in refluxing pyridine to diphenylacetylene:[1]
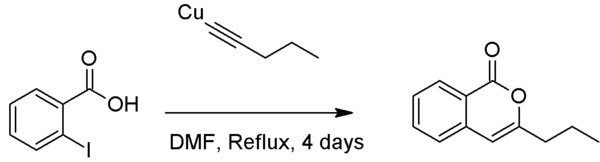
Unlike the Sonogashira coupling, the Castro–Stephens coupling can produce heterocyclic compounds when a nucleophilic group is ortho to the aryl halide, although this typically requires use of dimethylformamide (DMF) as solvent.[7][8]
References
- ^ a b c Stephens, R. D.; Castro, C. E. (1963). "The Substitution of Aryl Iodides with Cuprous Acetylides. A Synthesis of Tolanes and Heterocyclics". J. Org. Chem. 28 (12): 3313–3315. doi:10.1021/jo01047a008.
- ^ a b Owsley, D. C.; Castro, C. E. (1972). "Substitution of Aryl Halides with Copper(I) Acetylides: 2-Phenylfuro[3,2-b]pyridine". Organic Syntheses. 52: 128. doi:10.15227/orgsyn.052.0128; Collected Volumes, vol. 6, p. 916.
- ^ Rosenmund, Karl W.; Struck, Erich (1919). "Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe" [The halogen bound to the ring carbon and its replacement by other substituents. I. Notice: Replacement of the halogen by the carboxyl group] (PDF). Ber. Dtsch. Chem. Ges. A/B (in German). 52 (8): 1749–1756. doi:10.1002/cber.19190520840.
- ^ von Braun, Julius; Manz, Gottfried (1931). "Fluoranthen und seine Derivate. III. Mitteilung" [Fluoranthene and its derivatives. III. Notification]. Justus Liebigs Ann. Chem. (in German). 488 (1): 111–126. doi:10.1002/jlac.19314880107.
- ^ Sonogashira, Kenkichi; Tohda, Yasuo; Hagihara, Nobue (1975). "A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines". Tetrahedron Lett. 16 (50): 4467–4470. doi:10.1016/s0040-4039(00)91094-3.
- ^ Sonogashira, Kenkichi (2002). "Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides". J. Organomet. Chem. 653 (1–2): 46–49. doi:10.1016/s0022-328x(02)01158-0.
- ^ Batu, Gunes; Stevenson, Robert (1980). "Synthesis of natural isocoumarins, artemidin and 3-propylisocoumarin". J. Org. Chem. 45 (8): 1532–1534. doi:10.1021/jo01296a044.
- ^ Castro, Charles E.; Havlin, R.; Honwad, V. K.; Malte, A. M.; Moje, Steve W. (1969). "Copper(I) Substitutions. Scope and Mechanism of Cuprous Acetylide Substitutions". J. Am. Chem. Soc. 91 (23): 6464–6470. doi:10.1021/ja01051a049.

![Castro–Stephens reaction {\displaystyle {\begin{aligned}{}\\{\ce {Cu-C{\equiv }C-R'}}+{\color {Red}{\ce {R-X}}}\ &{\ce {->[{\ce {pyridine}}]CuX}}+{\color {Red}{\ce {R}}{-}}{\ce {C{\equiv }C-R'}}\\{\color {Red}{\ce {X}}}&={\color {Red}{\ce {I,Br,Cl}}}\\{\color {Red}{\ce {R}}}&={\color {Red}{\ce {Ar}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bb20e472e272f9d2dd48aff4835d778853ac6182)