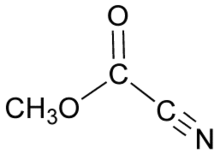
Methyl cyanoformate
| |
| Names | |
|---|---|
| IUPAC name
Methyl cyanoformate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.826 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H3NO2 | |
| Molar mass | 85.06 |
| Appearance | colorless liquid |
| Density | 1.072 g/cm3 |
| Boiling point | 100 to 101 °C (212 to 214 °F; 373 to 374 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl cyanoformate is the organic compound with the formula CH3OC(O)CN. It is used as a reagent in organic synthesis as a source of the methoxycarbonyl group,[1] in which context it is also known as Mander's reagent. When a lithium enolate is generated in diethyl ether or methyl t-butyl ether (but not THF), treatment with Mander's reagent will selectively afford the C-acylation product.[2] Thus, for enolate acylation reactions in which C- vs. O-selectivity is a concern, methyl cyanoformate is often used in place of more common acylation reagent like methyl chloroformate.
It is notoriously known for being an ingredient in Zyklon A, a predecessor to Zyklon B, the brand name of a German gas pesticide that was used during the Holocaust.[citation needed]
References
- ^ Simon R. Crabtree, W. L. Alex Chu, Lewis N. Mander "C-Acylation of Enolates by Methyl Cyanoformate: An Examination of Site- and Stereoselectivity"Synlett 1990; 1990: 169–170. doi:10.1055/s-1990-21025
- ^ Crabtree, Simon R.; Chu, W. L. Alex; Mander, Lewis N. (1990). "C-Acylation of Enolates by Methyl Cyanoformate: An Examination of Site- and Stereoselectivity". Synlett. 1990 (3): 169–170. doi:10.1055/s-1990-21025. ISSN 0936-5214.
