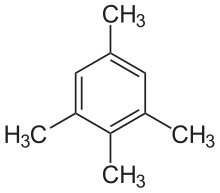
Isodurene
Appearance
| |
| Names | |
|---|---|
| IUPAC name
1,2,3,5-tetramethylbenzene
| |
| Other names
Isodurene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.653 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14 | |
| Molar mass | 134.22 |
| Appearance | colorless liquid |
| Density | 0.89 g/cm3 |
| Melting point | −23.7 °C (−10.7 °F; 249.5 K) |
| Boiling point | 198 °C (388 °F; 471 K) |
| 27.9 mg/L | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable |
| GHS labelling: | |

| |
| Warning | |
| H315, H319 | |
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |
| Flash point | 63.3 °C (145.9 °F; 336.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isodurene or 1,2,3,5-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Isodurene is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene).[1]
Production
Industrially, isodurene can be isolated from the reformed fraction of oil refineries. It may also be produced by methylation of toluene, xylenes and trimethylbenzenes.[1]
References
- ^ a b Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2002). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227. ISBN 978-3527306732.
