Monobutyl phthalate
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Butoxycarbonylbenzoic acid[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.580 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H14O4 | |
| Molar mass | 222.240 g·mol−1 |
| Appearance | White solid |
| Melting point | 73.5 °C (164.3 °F; 346.6 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H360 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,000 mg kg−1 (mouse, intraperitoneal)[1][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
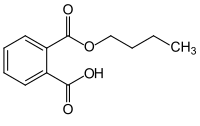
Monobutyl phthalate (MBP) is an organic compound with the condensed structural formula CH3(CH2)3OOCC6H4COOH. It is a white solid that features both an butyl ester group and a carboxylic acid group. It is the major metabolite of dibutyl phthalate. Like many phthalates, MBP has attracted attention as a potential endocrine disruptor.[3]
MBP is also the secondary metabolite of butyl benzyl phthalate, less than monobenzyl phthalate (MBzP). It hydrolyses to phthalic acid and 1-butanol.[4]
References
- ^ a b c d e f g "Monobutyl phthalate". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. January 4, 2020. Retrieved January 10, 2020.
- ^ Chambon, Pierre; Riotte, Maurice; Daudon, Marc; Chambon-Mougenot, Renée; Bringuier, Janine (1971). "Etude du métabolisme des phtalates de dibutyle et de diéthyle chez le Rat" [Metabolism of dibutyl and diethyl phthalates in the rat]. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, Série D: Sciences Naturelles (in French). 273 (22): 2165–2168. PMID 5003086.
- ^ Hu Y, Dong C, Chen M, Chen Y, Gu A, Xia Y, Sun H, Li Z, Wang Y. "Effects of monobutyl phthalate on steroidogenesis through steroidogenic acute regulatory protein regulated by transcription factors in mouse Leydig tumor cells". Journal of Endocrinological Investigation. 38 (8): 875–884. doi:10.1007/s40618-015-0279-6.
- ^ Huang, Jingyu; Nkrumah, Philip N.; Li, Yi; Appiah-Sefah, Gloria (2013). "Chemical Behavior of Phthalates Under Abiotic Conditions in Landfills". Reviews of Environmental Contamination and Toxicology. Vol. 224. New York, NY: Springer Science+Business Media. pp. 39–52. doi:10.1007/978-1-4614-5882-1_2. ISBN 9781461458814. PMID 23232918.
