Staggered conformation
Appearance
This article provides insufficient context for those unfamiliar with the subject. (March 2011) |

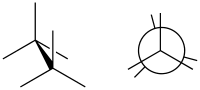
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX–Ydef in which the substituents a, b, and c are at the maximum distance from d, e, and f. This requires the torsion angles to be 60°.[1]
Such a conformation exists in any open chain single chemical bond connecting two sp3-hybridised atoms, and is normally a conformational energy minimum. For some molecules such as those of n-butane, there can be special versions of staggered conformations called gauche and anti; see first Newman projection diagram in Conformational isomerism.
- Crystal structures
- The staggered/eclipsed configurations distinguish different crystalline structures of eg. cubic/hexagonal boron nitride, and diamond/lonsdaleite.
See also
References
- ^ Eliel, Ernest L.; Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. Wiley. p. 1207. ISBN 978-0-471-01670-0.
