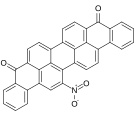
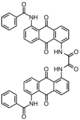
Vat dye
Vat dyes are a class of dyes that are classified as such because of the method by which they are applied. Vat dyeing is a process that refers to dyeing that takes place in a bucket or vat. The original vat dye is indigo, once obtained only from plants but now often produced synthetically.[1]
Materials suited for vat dyeing
Although almost all dyeing can be done in a vat, the term vat dye is used to describe a chemical class of dyes that are applied to cellulosic fibre (i.e.. cotton) using a redox reaction as described below. Because of the use of caustic soda, and the very high pH of the dye bath in the dyeing process, wool cannot be dyed using vat dyestuffs. This is because wool is soluble in caustic soda solutions. Instead, it is possible to dye wool at room temperatures with indigo (vat blue 1) and other low substantive vat dyes using soda ash as the alkali source with very little strength loss. Vat red 10, vat violet 13 and vat orange 1 can be applied in this manner as well.[2]
Dyeing process
Vat dyes characteristically require a reducing agent to solubilize them. The most common reducing agent is sodium dithionite (Na2S2O4), which converts the dye to its "leuco" form that is soluble. Once attached to the fabric, the leuco dye is then oxidized to the insoluble state which is intensely colored. Chemical reactions such as oxidation, reduction, pH control are often necessary; even the dissolution process necessitates measuring out appropriate quantities of caustic soda and sodium hydrosulphite in order to achieve reduction. The dye is soluble only in its reduced form. The fiber is immersed repeatedly in this oxygen-free dyebath, then exposed to the air, whereupon the water-soluble reduced form changes color as oxygen turns it to the water-insoluble form. For these reasons, vat dyes are less suitable than fiber-reactive dyes for amateur use.
Indigo is an example of this dye class: it changes from yellow, in the dyebath, to green and then blue as the air hits it.
Not all vat dyeing is done with vat dyes.
Properties
The vat dyes have high color fastness, which is uncommon in other dye classes. On the other hand, vat dyes tend to have poor rubbing fastness, but this can be mitigated with special treatments to the fabric. Indigo is subject to major crocking (i.e., rubbing the dye off onto other items) unless it is applied carefully. This means that dipping many times in a weaker dyebath is more preferred than to dip once in a stronger dyebath.
Light-oxidized vat dyes
Inkodye is a type of vat dye that uses light rather than oxygen to "fix" the dye, with a wide variety of possible effects. These dyes, which are chemically similar to vat dyes, are developed by light instead of being applied in an oxygen-free bath and being developed in the fabric by exposure to oxygen. Inkodyes are true dyes, not fabric paints. A dye itself attaches to the fabric; fabric paint includes a glue-like binder, which imparts a stiffer feeling to the fabric.
Chemical structures
. For example, vat blue 2 and 3 are halogenated or methylated derivatives and so are several violets. Many other vat dyes are derivatives of anthroquinones.[3]
- Blue vat dyes
-
Vat Blue 21
-
Vat Blue 25
-
Vat Blue 26
-
Vat Blue 30
-
Vat Blue 64
- Green vat dyes
-
Vat Green 3
-
Vat Green 8
-
Vat Green 11
-
Vat Green 12
- Orange vat dyes
-
Vat Orange 2
-
Vat Orange 9
-
Vat Orange 15
-
Vat Orange 17
- Violet vat dyes
-
Vat violet 15
-
Vat Violet 18
- Red vat dyes
-
Vat Red 10
-
Vat Red 13
-
Vat Red 18
-
Vat Red 28
- Brown vat dyes
-
Vat Brown 1
-
Vat Brown 3
-
Vat Brown 45
- Black vat dyes
-
Vat Black 25
-
Vat Black 27
-
Vat Black 29
- Yellow vat dyes
-
Vat Yellow 10
-
Vat Yellow 12
-
Vat Yellow 20
-
Vat Yellow 28
References
- ^ Booth, Gerald (2000). Dyes, General Survey. Wiley-VCH. doi:10.1002/14356007.a09_073.
- ^ The Chemistry of Vat Dyes by Dianne Epps
- ^ Bien, H.-S.; Stawitz, J.; Wunderlich, K. "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)
Imagery on Fabric by Jean Ray Laury