Sodium tetrasulfide
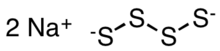
| |
| Names | |
|---|---|
| IUPAC name
Sodium tetrasulfide
| |
| Other names
disodiumtetrasulphide, sodium sulfide
| |
| Identifiers | |
| ECHA InfoCard | 100.031.628 |
| EC Number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| Na2S4 | |
| Molar mass | 174.24g/mol |
| Appearance | Dark red, slightly viscous liquid or yellow crystalline powder |
| Density | 1.268 g/cm3 at 15.5 °C |
| Melting point | 275 °C (527 °F; 548 K) |
| Soluble in water | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Stable at room temperature, but can be explosive when heated. Reactions with acids or oxidative agents will create gaseous byproducts that would be hazardous if inhaled. |
| NFPA 704 (fire diamond) | |
| Not applicable | |
| Safety data sheet (SDS) | [1] [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a yellow-orange solid that dissolved with hydrolysis in water.[2] They are precursors to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.
Synthesis and structure
It is produced through the reaction between elemental sulfur and sodium hydrosulfide in alcoholic solution:[3]
- 2NaSH + 4 S → Na2S4 + H2S
The polysulfide anions adopt zig-zag chains of sulfur atoms. The S-S distances are about 2.05 Å and the S-S-S-S dihedral angles are around 90°.[4]
Reactions and applications
Upon treatment with acid, it is converted to hydrogen sulfide and elemental sulfur. Treatment with alkylating agents gives organic polysulfides. In one commercial application, it is used to produce the cross-linking agent bis(triethoxysilylpropyl)tetrasulfide:[5]
- Na2S4 + 2 ClC3H6Si(OEt)3 → S4[C3H6Si(OEt)3]2 + 2 NaCl
Sometimes as a mixture with other polysulfides, sodium tetrasulfide is used to produce the polymer called thiokol. The reaction involves alkylation with ethylene chloride:
- Na2S4 + C2H4Cl2 → 1/n (C2H4)Sx]n + 2 NaCl
These materials, which have the approximate formula (C2H4)Sx]n (x ~ 4), are highly resistant to degradation by solvents and acids.[6]
References
- ^ "Safety Data Sheet, Sodium Tetrasulfide" (PDF). Pfaltz & Bauer.
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 365.
- ^ D. R. Brush (2000). "Sodium Sulfides". Kirk-Othmer Encyclopedia of Chemical Technology. Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1915040902211908.a01. ISBN 0471238961.
- ^ R. Tegman "The crystal structure of sodium tetrasulphide, Na2S4" Acta Crystallogr. (1973). B29, 1463-1469 doi:10.1107/S0567740873004735
- ^ Thurn, Friedrich; Meyer-Simon, Eugen; Michel, Rudolf "Verfahren zur Herstellung von Organosiliziumverbindungen (Continuous manufacture of bis[3-(triethoxysilyl)propyl] tetrasulfide)" Ger. Offen. (1973), DE 2212239 A1 19731004.
- ^ Sulfides, Polysulfides, and Sulfanes" in Ullmann's Encyclopedia of Industrial Chemistry Ludwig Lange and Wolfgang Triebel, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_443

