Condensation reaction
A condensation reaction is a class of organic addition reaction that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation).[1] The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.[2] It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.[3]
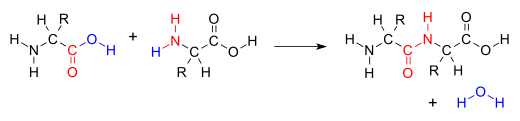
Many variations of condensation reactions exist, common examples include the aldol condensation, Claisen condensation, Knoevenagel condensation, and the Dieckman condensation (intramolecular Claisen condensation).[4]
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- ^ Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700.
- ^ "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- ^ Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
