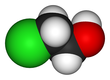
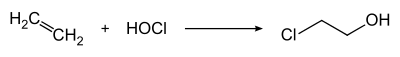2-Chloroethanol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chloroethan-1-ol[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 878139 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.146 | ||
| EC Number |
| ||
| 25389 | |||
| KEGG | |||
| MeSH | Ethylene+Chlorohydrin | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1135 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5ClO | |||
| Molar mass | 80.51 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Faint, ether-like | ||
| Density | 1.201 g·mL−1 | ||
| Melting point | −62.60 °C; −80.68 °F; 210.55 K | ||
| Boiling point | 127–131 °C; 260–268 °F; 400–404 K | ||
| Miscible[3] | |||
| log P | −0.107 | ||
| Vapor pressure | 700 Pa (at 20 °C) | ||
Refractive index (nD)
|
1.441 | ||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−1.1914 MJ·mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H226, H300, H310, H330 | |||
| P260, P280, P284, P301+P310, P302+P350 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 5–16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LC50 (median concentration)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (16 mg/m3) [skin][3] | ||
REL (Recommended)
|
C 1 ppm (3 mg/m3) [skin][3] | ||
IDLH (Immediate danger)
|
7 ppm[3] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
2-Chloroethanol is a chemical compound with the formula HOCH2CH2Cl and the simplest chlorohydrin. This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional groups.
Synthesis and applications
2-Chloroethanol is produced by treating ethylene with hypochlorous acid:
2-Chloroethanol was once produced on a large scale as a precursor to ethylene oxide:
This application has been supplanted by the more economic direct oxidation of ethylene. Otherwise chloroethanol is still used in a number of specialized applications.[6] Several dyes are prepared by the alkylation of aniline derivatives with chloroethanol.[7] It is a building block in the production of pharmaceuticals, biocides and plasticizers. It is also used for manufacture of thiodiglycol. It is a solvent for cellulose acetate and ethyl cellulose, textile printing dyes, in dewaxing, refining of rosin, extraction of pine lignin, and the cleaning of machines.
Environmental aspects
Chloroethanol is a metabolite in the degradation of 1,2-dichloroethane. The alcohol is then further oxidized via chloroacetaldehyde to chloroacetate. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane are processed annually as a precursor to vinyl chloride.[8]
Safety
2-Chloroethanol is toxic with an LD50 of 89 mg/kg in rats. Like most organochlorine compounds, chloroethanol combusts to yield hydrogen chloride and phosgene.
In regards to dermal exposure to 2-chloroethanol, the Occupational Safety and Health Administration has set a permissible exposure limit of 5 ppm (16 mg/m3) over an eight-hour time-weighted average, while the National Institute for Occupational Safety and Health has a more protective recommended exposure limit of a 1 ppm (3 mg/m3) exposure ceiling.[9]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[10]
References
- ^ a b
Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 29. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
For example, the omission of the locant '1' in 2-chloroethanol, while permissible in general usage, is not allowed in preferred IUPAC names, thus the name 2-chloroethan-1-ol is the PIN.
- ^ a b c d e f g h i j k l Depositor-supplied synonyms for CID 34
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0268". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Ethylene chlorohydrin". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "New Environment Inc. - NFPA Chemicals".
- ^ Liu, Gordon Y. T.; Richey, W. Frank; Betso, Joanne E.; Hughes, Brian; Klapacz, Joanna; Lindner, Joerg (2014). "Chlorohydrins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_565.pub2. ISBN 978-3527306732.
- ^ Raue, Roderich; Corbett, John F. (2002). "Nitro and Nitroso Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_383. ISBN 978-3527306732.
- ^ Janssen, D. B.; van der Ploeg, J. R.; Pries, F. (1994). "Genetics and Biochemistry of 1,2-Dichloroethane Degradation" (PDF). Biodegradation. 5 (3–4): 249–57. doi:10.1007/BF00696463. PMID 7765836.
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards
- ^ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF). Code of Federal Regulations (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.[failed verification]